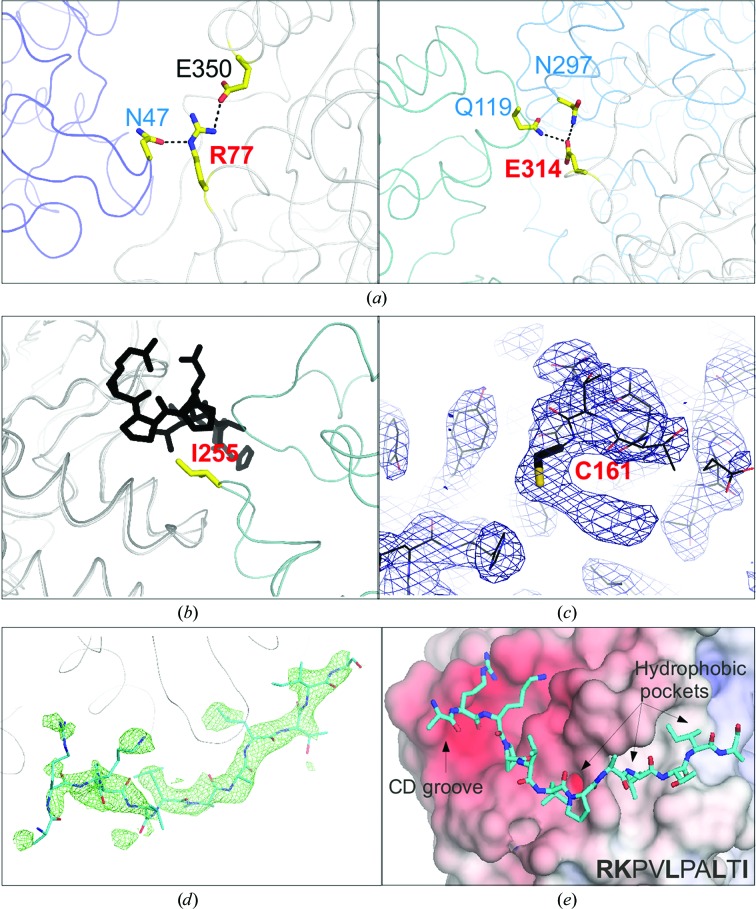
Figure 1.
Surface engineering of ERK2 to interfere with ‘undesired’ crystal packing. (a) Arg77 forms hydrogen bonds with Asn47 from a symmetry mate in the crystal (left panel) and Glu314 interacts with Gln119 and with Asn297 from two ERK2 WT molecules (right panel). (b) The side chain of Ile255 from an ERK2 symmetry mate (coloured teal) occupies the hydrophobic groove in the apo ERK2_AA structures. The superimposed ERK–pepDCC complex structure (PDB entry 3o71; Ma et al., 2010 ▶), shown in dark grey (MAPK) and black (pepDCC), on ERK2_AA demonstrates that this type of crystal packing is incompatible with D-motif peptide binding. (c) The 2F o − F c electron-density map contoured at 1σ for the final apo ERK2_AAG structure shows strong and continuous density for the side chain of Cys161. This indicates adduct formation with β-mercaptoethanol at this cysteine residue. (d) F o − F c simulated-annealing OMIT map contoured at 2σ for the ERK2–pepMKK2 complex. (e) Crystal structure of the ERK2–pepMKK2 complex. The ERK2 surface is coloured according to its electrostatic potential (red, negative; blue, positive).