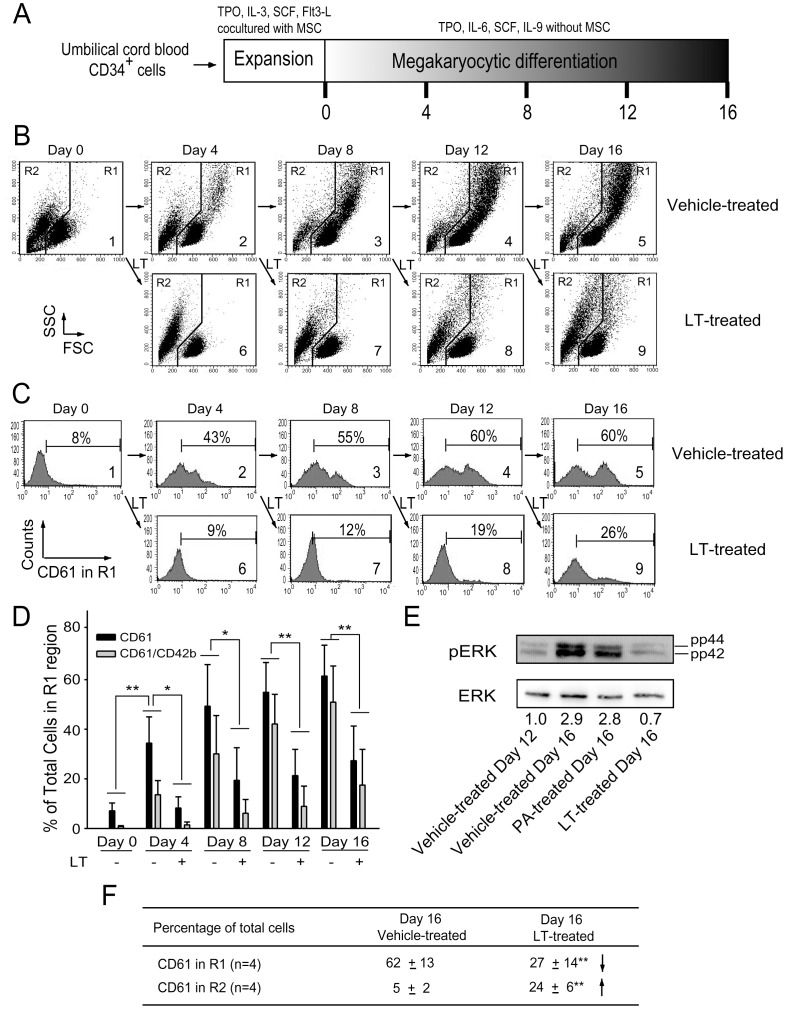
Figure 2. Suppressive effect of LT on in vitro megakaryocytic differentiation.
(A) The experiment outline of in vitro megakaryocytic differentiation using human cord blood-derived CD34+ cells. Cells were firstly expanded on mesenchymal stem cell (MSC) monolayer and then subjected to a 16-day course of differentiation (0–16 day), in which LT and vehicle (diluents: cell-culture medium) were treated to various groups by day 0, 4, 8, and 12, respectively (B1–B9; C1–C9). Four days after LT treatments, megakaryocytic surface marker CD61 (GPIIIa; total megakaryocytes) and CD42b (GPIb; mature megakaryocytes) of each groups were then analyzed by flow cytometry on days 4, 8, 12, and 16, respectively. Flow cytometry analysis of the cell size (FSC) and cell granularity (SSC) at various time points are shown (B). The percentage of CD61+ cells in R1 regions (B) is illustrated in (C). Quantitative results on the percentage of CD61+ and CD61+/CD42b+ cells in R1 regions at different differentiation time points are indicated (D). The entire population of R1+ R2 cells was defined as 100%. Data are reported as mean ± standard deviation (SD) and represent 4 independent experiments. The images for Western blot of phosphorylated-ERK (pERK) and total ERK are shown (E). Relative gel intensities (fold change) after normalized with respective total ERK levels are indicated below the blot images, in which the Day 12 group was normalized to one fold. Summarized events occurred on day16 were shown (F). **p<0.01 compared to indicated groups.