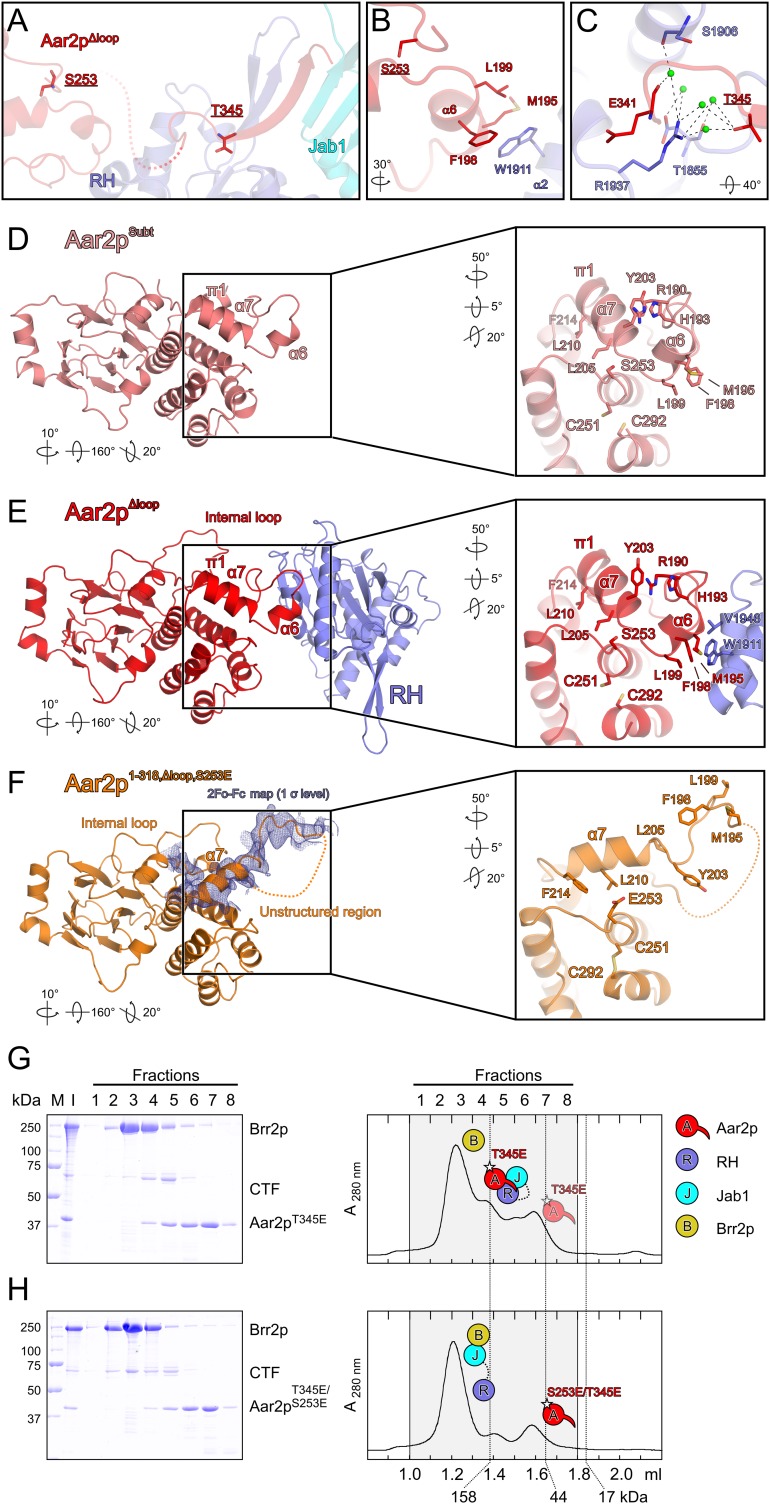
Figure 6.
Effect of phospho-mimetic mutations in Aar2p on Brr2p–CTF complex formation. (A) Position of two Aar2p phosphorylation sites, S253 and T345, in the Aar2pΔloop–RH–Jab1 complex structure. Colors and labels are as in Figure 1C. (B) Close-up view of Aar2p residue S253 (underlined) and its environment. (C) Close-up view of Aar2p residue T345 (underlined) and its environment. (D–F) Comparison of the structures of Aar2pSubt (Weber et al. 2011), the Aar2pΔloop–RH–Jab1 complex, and Aar2p1–318,Δloop,S253E. (Blue mesh in F) 2Fo − Fc electron density covering the α7 region contoured at the 1σ level. Rotation symbols indicate the views relative to Figure 1B. Insets show the α6/α7 region in these structures, which is remodeled upon introducing the S253E mutation. Rotation symbols indicate the views relative to the overviews. (G,H) Gel filtration analysis probing the interaction of Aar2p bearing phospho-mimetic mutations with CTF and Brr2p. Details and labels are as in Figure 2. Icons representing the proteins are defined in the top right. All rotations indicated are relative to Figure 1B. (G) Aar2pT345E still binds CTF and excludes Brr2p. (H) The Aar2pS253E/T345E variant with two phospho-mimetic mutations no longer interferes with Brr2p–CTF complex formation.