Abstract
Objectives
Carotid body tumours (CBTs) are rare vascular neoplasms originating in paraganglionic cells of the carotid bifurcation. The aim of this study was to review all patients diagnosed with CBTs in Northern Ireland.
Methods
A retrospective review was performed of all patients who had CBTs treated at our institutions between 1987 and 2009. Patient demographics, clinical symptomatology, investigative modality, therapeutic intervention, pathological analysis and long-term outcomes were assessed.
Results
Twenty-nine patients were identified with 33 CBTs and three glomus intravagale tumours (GITs). Six patients had bilateral CBTs (21%), one of whom had a synchronous GIT. Twenty-six patients underwent a total of 30 operative procedures for the resection of 28 CBTs and 3 GITs. Conventional operative treatment included subadventitial tumour excision. A vascular shunt facilitated arterial reconstruction following the removal of seven (23%) tumours and on six of these occasions (19%) continuity was restored with an interposition vein graft. For access the external carotid artery was ligated during the removal of four tumours (13%). Two tumours were considered malignant. No peri-operative mortalities were recorded. Immediate complications included peri-operative stroke secondary to an occluded vein graft (n=1), requirement of tracheostomy (n=2), emergency haematoma drainage (n=2) and transient cranial nerve damage (n=8). Late complications included pseudoaneurysm of vein graft with subsequent stoke (n=1), permanent cranial nerve damage (n=9), Horner’s syndrome (n=1) and an asymptomatic vein graft occlusion (n=1). One patient had tumour recurrence two years post-operatively and died due to pulmonary metastases. Two other patients died of unrelated causes. All other patients remain well with no evidence of tumour recurrence at mean followup of 1801 days (range 159-9208 days).
Conclusion
Our long-term experience is comparable with other reported case series where surgical intervention conferred a long-term survival advantage despite associated cranial nerve co-morbidities.
Keywords>: Carotid Body, Complications, Outcome, Surgery, Tumour
INTRODUCTION
Carotid body tumours (CBTs) are rare vascular neoplasms originating in the paraganglionic cells of the carotid bifurcation. They have a reported incidence between 0.06 and 3.33 per 100,000 patients 1, 2. Male and female distribution is equal except at high altitude where females appear to predominate 3, 4. The second commonest type of cervical paraganglioma is a glomus intravagale tumour (GIT), which is derived from closely adjacent paraganglionic tissue located on the vagus nerve 2.
Clinically, CBTs typically present as a non-tender, rubbery, pulsatile mass. Classically, the mass can be displaced laterally but not vertically, due to carotid artery adherence, which is known as a positive Fontaine sign. Diagnosis is commonly confirmed by duplex ultrasound, computerised tomography (CT), magnetic resonance imaging (MRI) and rarely conventional angiography 5.
Although technically challenging, surgery remains the only definitive treatment 1, 2. Mathews (1915) remarked that ‘this rare tumour presents unusual difficulties to the surgeon and should one encounter it without suspecting the diagnosis, the experience will not be forgotten’ 6. Since the initial reports of peri-adventitial dissection by Gordon-Taylor (1940), modern methodologies, including intra-luminal carotid artery shunting and carotid arterial reconstruction with autologous vein grafts or prosthetic grafts if necessary, have dramatically reduced the most significant peri-operative complications of stroke and death 1, 2, 7, 8. Other early local complications encountered include the risk of bleeding and airway compromise, loss of baroceptor function and neurovascular damage. Longer-term CBT complications include recurrence, metastatic dissemination, graft pseudoaneurysm or occlusion and permanent cranial nerve palsies 9-12. As a result graft surveillance is recommended 13.
Histopathological analysis is a poor predictor of malignant potential. Malignancy is therefore defined only by the presence of distant metastases 14. Although 95% of all CBTs are benign, they remain locally aggressive tumours with growth rates of 2cm every five years, which can lead to localised mass effects or neurological dysfunction due to pressure or infiltration 1, 6.
Ten-percent of CBT cases will have a familial trait 15. Autosomal dominance may be associated with variable penetrance where the oncogenes c-myc, bcl-2 and c-jun have been implicated. Succinate dehydrogenase complex subunits B, C and D (SDHB, SDHC and SDHD) gene mutations are associated with genetic susceptibility. SDHB and SDHD, the most commonly implicated, also predispose to pheochromocytoma while SDHD gene mutations have been shown to correlate with the development of multiple CBTs. Approximately 30% of these familial tumours will be bilateral. Therefore, clinical surveillance combined with radiological imaging remains important for early identification of contra-lateral tumours 17.
The aim of this study was to review all patients treated by vascular surgeons in all tertiary referral centres for CBTs in Northern Ireland over a 22-year period and to compare our experience with published evidence.
METHODS
A retrospective case note review of all patients who had CBTs managed in our institutions between 1987 and 2009 was completed. To identify patients, pathology archival databases in laboratories related to each referral centre were searched for all diagnoses of CBT and theatre log books were explored for operative procedures on CBTs. Data collated into a predefined database included age, sex, presenting symptoms, pre-operative investigations, use of pre-operative embolisation, operative details including tumour size, Shamblin classification, need for arterial sacrifice or reconstruction, post-operative morbidity, pathological assessment, use of radiotherapy and long-term follow-up specifically with regards subsequent recurrence and development of disseminated malignancy.
RESULTS
Clinical Presentation
Twenty-nine patients were identified with a total of 33 CBTs and three GITs. There were 14 male and 15 female patients with a mean age of 49-years (range 16-85 years). Twenty tumours were located on the right side and 16 on the left. Six patients had bilateral CBTs (21%). One of these patients had a synchronous GIT. There were four cases of confirmed familial disease (14%) (Table 1).
Table 1.
Clinical presentations for the 29 patients in our series.
| Number | % | |
|---|---|---|
| Clinical Presentation | ||
| Painless neck mass | 19 | 65.5 |
| Painful neck mass | 5 | 17.2 |
| Collapse | 1 | 3.4 |
| Pre-auricular pain | 1 | 3.4 |
| Familial surveillance | 1 | 3.4 |
| Identified on follow up | 1 | 3.4 |
| Unavailable | 1 | 3.4 |
Investigations
Investigative modalities for the 29 patients included routine ultrasound (n=14), duplex ultrasound (n=5), computed tomography (n=18), magnetic resonance imaging (n=9) (Figure 1), radionuclide perfusion scan (n=6) and percutaneous angiography (n=11). Although percutaneous angiography and radio-isotope scans were commonly performed at the beginning of our series, ultrasound is now advocated as a first-line diagnostic or screening modality. Computed tomography and more recently magnetic resonance imaging are now used during pre-operative planning.
Fig 1.
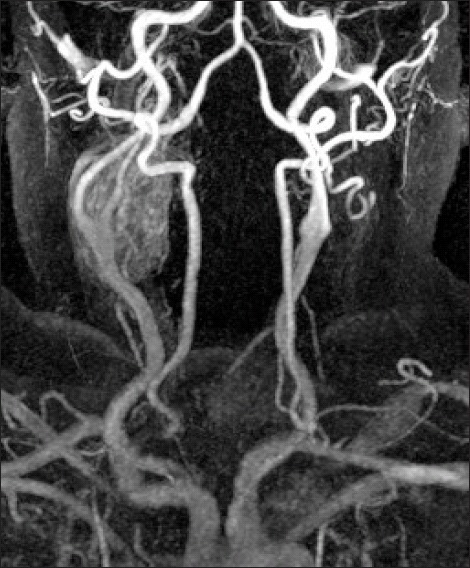
Magnetic resonance angiogram (MRA) with intravenous gadolinium showing an avidly enhancing 4 x 3.5 x 2.5cm right CBT with multiple small blood vessels within (Note the characteristic splaying of the carotid bifurcation with the external carotid artery bowing over the tumour and the internal carotid artery slightly narrowed in calibre as it passes through)
Other investigations included a PET scan which was performed to investigate tumour dissemination in a patient with previously diagnosed bilateral CBTs. Five non-diagnostic (C1) and one normal (C2) fine needle aspirations were recorded during the investigation of neck masses prior to referral to our units. Two patients also had incidental CBTs diagnosed during attempts at open biopsy of neck masses in other surgical units.
Pre-operative Treatment
Pre-operative embolisation was performed in two patients with successful occlusion of the main feeding branch of the right parapharyngeal artery four days pre-operatively in the first patient (F, 61). Despite two attempts in the other patient (M, 45), pre-operative embolisation served to increase collateral vasculature and was therefore deemed unsuccessful. No complications were caused by embolisation in either patient.
Surgical Intervention
Surgical intervention was not considered appropriate in three patients because of medical co-morbidities. One patient with bilateral CBTs was not considered for contra-lateral CBT surgery. A total of 26 patients underwent surgical excision of 28 CBTs and three GITs.
Shamblin classification demonstrated six grade I, five grade II, nine grade III and eleven unclassified tumours (Table 2). Surgery was performed by three experienced vascular surgeons using an oblique lateral neck incision along the anterior border of the sternocleidomastoid muscle followed by careful dissection to expose the carotid vessels characteristically splayed by the associated CBT (Figure 2). Control of the external, internal and common carotid arteries along with any major branches was completed using vascular sloops. The blood supply to CBTs generally arises initially from the external carotid artery and this has important implications in terms of obtaining exposure and control of this vessel. The glossopharyngeal, vagus, hypoglossal, ansa cervicalis, recurrent laryngeal, accessory and superior laryngeal nerves were protected when identified. A peri-adventitional caudal-cranial dissection was performed along the relatively avascular “white line” plane using bipolar diathermy for haemaostasis. Where possible, the tumour was subsequently enucleated without disturbing either the carotid vessels or cranial nerves (Figure 3). The external carotid artery was preserved where possible. Conventional subadventitial tumour excision was successful for the excision of 20 tumours (65%).
Table 2.
Shamblin classification - Definitions and corresponding patient data from our series.
| Shamblin Classification | Definition | Number | % |
|---|---|---|---|
| I | Small with minimal arterial attachment | 6 | 19.4% |
| II | Moderate arterial attachment partially surrounding carotids | 5 | 16.1% |
| III | Encasement of carotid bifurcation | 9 | 29.0% |
| Unclassified | Surgeon didn’t specify grade | 11 | 35.5% |
Fig 2.
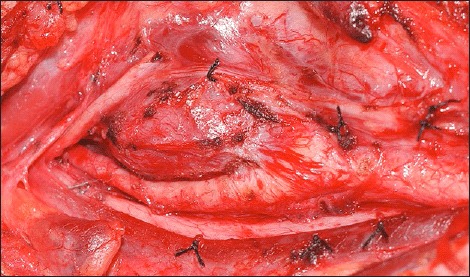
Intra-operative exposure of a right-sided CBT which has splayed the carotid bifurcation
Fig 3.

Undisturbed carotid arteries following excision of the right-sided CBT from figure 2 using the standard peri-adventitional dissection.
Intraluminal shunting and carotid artery reconstruction were performed for the removal of seven (23%) tumours where the tumour could not be enucleated without excision of a segment of carotid artery. Six of these tumours (19%) required en-bloc resection of the carotid bifurcation with continuity restored with an interposition long saphenous vein graft. For either access reasons or haemostasis, the external carotid artery was ligated in three more cases and during the removal of a total four tumours (13%). The internal carotid artery was preserved in all cases.
Complications
Early local complications included cranial nerve injuries in 17 patients where nine patients had multiple cranial nerve injuries. These included nine isolated hypoglossal, five facial and three accessory nerve injuries. Transient dysphagia was observed in seven patients with two patients requiring temporary nasogastric and percutaneous endoscopic gastrostomy (PEG) feeding respectively. Early systemic complications included one peri-operative stroke presenting with hemiparesis secondary to an occluded vein graft, which was treated with graft thrombectomy followed by partial resolution of symptoms. There were no peri-operative deaths (<30 days) (Table 3).
Table 3.
Early and late post-operative complications (CNI — Cranial nerve injury).
| Number | % | |
|---|---|---|
| Early Complications | ||
| Immediate | ||
| Airway obstruction | 2 | 6.7 |
| Local | ||
| Haematoma | 2 | 6.7 |
| Wound infection | 1 | 3.3 |
| Transient CNI | 8 | 26.7 |
| Vein graft occlusion | 1 | 3.3 |
| Systemic | ||
| Stroke | 1 | 3.3 |
| Late Complications | ||
| Stroke | 1 | 3.3 |
| Vein graft occlusion | 1 | 3.3 |
| Vein graft pseudoaneurysm | 1 | 3.3 |
| Permanent CNI | 9 | 30.0 |
| Horner's syndrome | 1 | 3.3 |
Long term local complications included nine patients with permanent cranial nerve damage comprising five patients who sustained injuries to multiple nerves including one superior laryngeal, one facial, two hypoglossal and two accessory nerve injuries. Only three patients required secondary procedures to address neurological symptoms which included thyroplasty (n=2) and teflon injection (n=1) for vocal cord paralysis while one also underwent pharyngoplasty for palatal paralysis. Long-term systemic complications included an asymptomatic vein graft occlusion diagnosed on follow-up surveillance treated conservatively. A further patient suffered a late stroke, seven years post-operatively, secondary to pseudoaneurysm of the vein graft which was treated with an endovascular stent (Table 3).
Pathological Analysis
Full pathological reports were available for 28 CBTs and three GITs. Mean tumour size was 3.72cm (range 1.8cm—8.0cm) (Figure 4). The majority of tumours (n=28, 90%) were well encapsulated and locally confined although three had an infiltrative growth pattern including perineural infiltration (n=1), vascular and capsular invasion (n=1) and regional lymph node spread (n=1). Histology demonstrated a typical nested growth pattern of monomorphic cells in most tumours with only focal pleomorphism and no significant mitotic activity. Tumour cells typically stained strongly with the neuroendocrine marker chromogranin A while sustentacular cells surrounding tumour cell nests stained with S100 (Figure 5).
Fig 4.

Gross specimen of the previously excised right-sided CBT (3 x 2.8 x 1.8cm, 6.5g)
Fig 5.
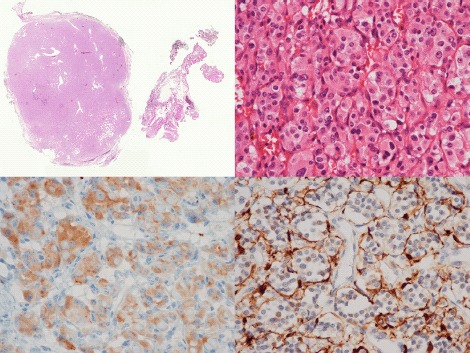
Low power view of a typical CBT, showing a thinly encapsulated, well-circumscribed mass, with some adherent wisps of connective tissue (H&E x 1) (Top left). High-power magnification displays a nested growth pattern of monomorphic cells with granular eosinophilic cytoplasm (H&E x 400) (Top right). Immunopositivity within tumour cell cytoplasm for the neuroendocrine marker chromogranin A (immunoperoxidase x 400). (Bottom left). Immunopositivity within surrounding sustentacular cells for S100 (immunoperoxidase x 400) (Bottom right).
Adjuvant Therapy
Three patients received post-operative radiotherapy for lymph node spread (F, 36), large tumour size with capsular invasion (M, 45) and local recurrence (F, 52). The remaining 23 surgical patients required no further post-operative treatment.
Clinical Outcome
Twenty-six patients remain well with no evidence of recurrence or disease dissemination at mean follow-up of 1801 days (range 159-9208 days). Two patients had metastatic disease and the first (F, 36) who had pathological evidence of nodal spread at the time of initial operation remains well without evidence of tumour recurrence at 9208 days follow-up. The second patient (F, 52), with perineural invasion in the initial resection specimen, developed local recurrence on computed tomography two years post-operatively. The recurrent tumour was successfully removed from the medial aspect of the distal end of a long saphenous vein anastomosis that had been fashioned at the time of the initial surgery following excision of a Shamblin class III tumour. This patient subsequently developed pulmonary metastases and despite a pulmonary wedge resection, died from progressive pulmonary metastases 4 years following her initial CBT surgery. The last of the three patients who received post-operative radiotherapy (M, 45) for capsular invasion remained well at 326 days until lost to follow-up. Two other patients died of unrelated causes. The overall CBT related mortality in our series was 3%.
DISCUSSION
All CBTs in Northern Ireland are managed by vascular surgeons, within the two main tertiary referral centres included in the study. Our study reports an incidence of CBTs in Northern Ireland of 0.08 per 100,000 people each year with a population prevalence equating to 1.5 per 100,000 people, which is comparable to the reported literature (Northern Ireland Statistics and Research Office). However, our 15% rate for familial tumours is relatively high compared to other regions with familial CBT rates around 10% 3, 5, 10, 12, 15, 16, 17, (Table 4).
Table 4.
Literature review of all previous CBT studies (Pts=patients with CBTs, Surg=patients managed surgically, M=Male / F=Female {*sex distribution figures for all paragangliomas highlighted}, Age=mean patient age, No.Tu=total numbers of tumours, Bilat=% bilateral tumours, Malig=% malignant tumours, Famil=% familial tumours, ECA Lig=% times ECA ligated, ICA lig=% times ICA ligated, ICAR=% times ICA reconstructed, CNI=% total cranial nerve injuries, TCNI=% temporary cranial nerve injuries, PCNI=% permanent cranial nerve injuries, CVA=% cerebrovascular accidents, Death=peri-operative mortality).
| Studies | Pts | Surg | M | F | Age | No. Tu | Bilat | Malig | Embol | Famil | ECAlig | ICAlig | ICAR | CNI | TCNI | PCNI | CVA | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Koskas et al 2009 | 36 | 36 | 14 | 22 | 44.4 | 39 | 3% | 0% | 3% | 5% | 56% | 23% | 72% | 46% | 26% | 3% | 0% | |
| Papaspyrou et al 2008 * | 38 | 36 | 39 | 81 | 42 | 46 | 18% | 3% | 5% | 10% | 3% | 25% | 23% | 4% | 0% | |||
| Makeieff et al 2008 | 52 | 52 | 17 | 35 | 43 | 57 | 6% | 2% | 0% | 12% | 26% | 2% | 9% | 42% | 35% | 7% | 2% | 0% |
| Van der Bogt et al 2008 | 94 | 94 | 44 | 50 | 41 | 111 | 61% | 2% | 0% | 64% | 1% | 3% | 38% | 15% | 23% | 0% | 0% | |
| Sajid et al 2007 | 95 | 95 | 32 | 63 | 55 | 95 | 18% | 4% | 18% | 19% | 18% | 1% | 1% | 1% | ||||
| Antonitsis et al 2006 | 13 | 12 | 8 | 41 | 14 | 8% | 0% | 79% | 0% | 25% | 0% | 8% | 54% | 54% | 0% | 0% | 0% | |
| Bakoyiannis et al 2006 | 11 | 11 | 8 | 3 | 35 | 12 | 9% | 0% | 0% | 8% | 25% | 25% | 0% | 0% | 0% | |||
| Kasper et al 2006 * | 20 | 20 | 13 | 12 | 51 | 25 | 25% | 52% | 12% | 32% | 4% | 52% | 40% | 12% | 0% | 0% | ||
| Knight et al 2006 | 16 | 15 | 4 | 12 | 68 | 16 | 7% | 7% | 0% | 0% | 0% | 0% | 0% | |||||
| Smith et al 2006 | 62 | 62 | 26 | 36 | 71 | 55% | 77% | 19% | 23% | 35% | 0% | 0% | ||||||
| Davidovic et al 2005 | 12 | 12 | 3 | 9 | 52 | 12 | 0% | 0% | 0% | 42% | 0% | 42% | 25% | 25% | 0% | 0% | 0% | |
| Luna-Ortiz et al 2005 | 66 | 46 | 2 | 64 | 50.2 | 69 | 5% | 0% | 0% | 0% | 11% | 7% | 6% | 49% | 12% | 38% | 4% | 0% |
| Heis et al 2003 | 9 | 8 | 4 | 5 | 48 | 9 | 0% | 0% | 0% | 0% | 13% | 25% | 25% | 0% | 0% | 0% | ||
| Patetsios et al 2002 | 29 | 28 | 10 | 19 | 43 | 34 | 17% | 10% | 0% | 25% | 29% | 46% | 29% | 17% | 0% | 4% | ||
| Dardik et al 2002 | 25 | 25 | 9 | 16 | 48.2 | 27 | 24% | 96% | 4% | 33% | 4% | 15% | 33% | 7% | 26% | 4% | 0% | |
| Persky et al 2002 * | 26 | 24 | 22 | 25 | 47 | 28 | 8% | 12% | 100% | 12% | 4% | 46% | 34% | 12% | 0% | 0% | ||
| Por et al 2002 | 7 | 7 | 2 | 5 | 43 | 8 | 14% | 0% | 38% | 50% | 75% | 25% | 50% | 13% | 0% | |||
| Huang et al 2001 | 30 | 30 | 0% | 17% | 27% | 0% | 0% | |||||||||||
| Plukker et al 2001 | 39 | 35 | 14 | 25 | 43 | 45 | 15% | 5% | 26% | 34% | 2% | 10% | 24% | 5% | 0% | |||
| Thabet et al 2001 | 16 | 16 | 11 | 5 | 42 | 18 | 13% | 6% | 6% | 6% | 44% | 6% | 6% | |||||
| Liapis et al 2000 | 18 | 16 | 7 | 11 | 45 | 18 | 0% | 11% | 19% | 25% | 25% | 0% | 0% | 0% | ||||
| Wang et al 2000 | 29 | 28 | 16 | 13 | 39.5 | 36 | 3% | 0% | 61% | 10% | 41% | 17% | 24% | 0% | 0% | |||
| Bastounis et al 1999 | 17 | 17 | 6 | 11 | 45 | 20 | 18% | 10% | 0% | 35% | 5% | 10% | 15% | 15% | 0% | 6% | 6% | |
| Rodriguez et al 1998 | 120 | 80 | 11 | 107 | 49 | 5% | 3% | 1% | 3% | 20% | 4% | 3% | ||||||
| Westerband et al 1998 | 31 | 31 | 15 | 16 | 48 | 32 | 3% | 3% | 19% | 29% | 26% | 13% | 6% | 0% | ||||
| Leonetti et al 1997 | 19 | 16 | 7 | 9 | 42.5 | 16 | 0 | 0% | 19% | 0% | 0% | 69% | 44% | 25% | 0% | 0% | ||
| Muhm et al 1997 | 24 | 19 | 10 | 14 | 51 | 28 | 17% | 4% | 42% | 13% | 36% | 9% | 26% | 5% | 0% | |||
| Litle et al 1996 | 21 | 21 | 8 | 13 | 46 | 22 | 24% | 50% | 23% | 5% | 18% | 45% | 27% | 18% | 5% | 0% | ||
| Mitchell et al 1996 * | 14 | 14 | 9 | 8 | 54.4 | 17 | 18% | 6% | 0% | 14% | 29% | 6% | 41% | 29% | 12% | 6% | 6% | |
| Matticari et al 1995 | 20 | 19 | 9 | 11 | 51.3 | 22 | 10% | 0% | 0% | 0% | 0% | 5% | 0% | 0% | ||||
| Netterville et al 1995 | 30 | 29 | 13 | 17 | 42 | 46 | 53% | 7% | 53% | 3% | 24% | 34% | 10% | 24% | 0% | 3% | ||
| Sanghvi et al 1993 | 20 | 20 | 13 | 7 | 21 | 5% | 0% | 0% | 15% | 45% | 5% | 0% | ||||||
| Rabl et al 1993 | 11 | 11 | 4 | 7 | 58.7 | 12 | 9% | 16% | 8% | 16% | 16% | 8% | 8% | 0% | 5% | |||
| La Muraglia et al 1992 | 17 | 17 | 5 | 12 | 44 | 19 | 12% | 0% | 58% | 37% | 0% | 11% | 16% | 11% | 5% | 6% | 0% | |
| Wax et al 1992 | 16 | 16 | 7 | 9 | 40 | 19 | 19% | 56% | 16% | 44% | 6% | 0% | ||||||
| Williams et al 1992 | 30 | 30 | 10 | 20 | 54 | 33 | 10% | 9% | 40% | 0% | 3% | 20% | 13% | 7% | 3% | 0% | ||
| Torres et al 1991 | 96 | 32 | 11 | 85 | 52.3 | 2% | 1% | 0 | 3% | |||||||||
| Yang et al 1991 | 27 | 27 | 44% | 30% | 15% | 2% | 7% | |||||||||||
| Hallett et al 1988 | 139 | 139 | 52 | 153 | 2% | 1% | 33% | 25% | 40% | 21% | 19% | 14% | 3% | |||||
| McPherson et al 1988 | 25 | 25 | 9 | 16 | 47 | 26 | 10% | 4% | 8% | 0% | 0% | 8% | 15% | 0% | 0% | |||
| Gaylis et al 1987 | 50 | 46 | 33 | 17 | 49 | 52 | 4% | 14% | 13% | 17% | 4% | 5% | ||||||
| Pachedo-ojeda 1988 | 19 | 19 | 5 | 14 | 52.5 | 20 | 5% | 0% | 0% | 11% | 10% | 0% | 10% | 26% | 11% | 15% | 5% | 0% |
| Dickinson et al 1985 | 32 | 25 | 10 | 22 | 41 | 37 | 14% | 0% | 27% | 0% | 15% | 40% | 20% | 20% | 4% | 0% | ||
| Lees et al 1981 | 39 | 37 | 18 | 21 | 49 | 43 | 10% | 15% | 5% | 10% | 15% | 17% | 5% | 2% | ||||
| Rosen et al 1981 | 27 | 24 | 14 | 13 | 30 | 11% | 8% | 7% | 8% | 33% | 33% | 17% | 16% | 0% | 4% | |||
| Farr 1980 | 43 | 43 | 31 | 31 | 46 | 44 | 2% | 7% | 0% | 18% | 18% | 16% | 9% | 14% | ||||
| Shamblin 1971 | 90 | 70 | 62 | 28 | 96 | 7% | 2% | 33% | 21% | 55% | 22% | 6% |
CBTs are often asymptomatic with most patients presenting with neck asymmetry or a distinct lump. If symptomatic, as shown in our study, neck pain is the most common complaint (17%). Although patients can present with the effects of functional tumours secreting histamine, serotonin, adrenaline and noradrenaline, this did not occur in our study 1, 5.
Non-invasive investigative modalities utilised in the work-up of patients with suspected CBT include duplex ultrasound, computerised tomography angiography and magnetic resonance angiography (MRA) 5, 17. Fine needle aspiration is rarely employed because of the risk of carotid injury or haemorrhage in these highly vascular tumours and open biopsy is clearly contraindicated due to the risk of catastrophic haemorrhage 5. We advocate duplex ultrasound as first line diagnostic or screening modality particular in patients with a positive family history 18. However, similar to other units, we now use MRA to follow-up CBT patients. MRA is safe, non-invasive, highly specific and sensitive for lesions involving the skull base or with a multicentric morphology 19 (Figure 1). Although invasive, routine angiography permits an accurate assessment of vascular anatomy, particularly carotid arterial neo-vascularisation, combined with intra-cerebral flow on the contralateral side, which is important to consider prior to intra-operative occlusion of the ipsilateral carotid circulation when excising the CBT [15].
Schick et al (1980) first described the use of pre-operative arterial embolisation which has been reported to decrease blood loss and subsequent transfusion rates whilst leading to potential reductions in tumour size by up to 25% if performed within 48-hours of surgery in medium to large CBTs with well-defined feeding vessels 13, 15, 17, 20. However, if surgery is delayed, revascularisation oedema combined with a localised inflammatory response can create difficulty with the periadventitial dissection 19, 21. Embolisation is also a time consuming process associated with the inherent risks of distal migration of the embolisation medium and a stroke incidence as high as 10% 17, 19-20. Other authors have described no effect on blood loss, transfusion requirements or duration of surgery following embolisation 22. Consistent with these conflicting results, use of pre-operative embolisation varies widely across the literature with rates between 0% and 100% (Table 4). Although we report a 7% use of pre-operative embolisation, which is low in comparison to the majority of studies, we experienced no complications secondary to the procedure such as transient ischaemic attacks and strokes 21-23.
Advances in endovascular surgery suggest the possibility of vascular exclusion of external carotid artery feeding branches to the tumour through deployment of covered stents. These limited case reports postulate that the use of endovascular stents, without the use of coils or intra-arterial gel foams, may potentially lower the risk of peri-procedural stroke 10. However, these techniques are rarely used and indeed were not performed in any of the 47 case series reviewed in Table 4. We advocate preservation of the carotid circulation and also report no endovascular external carotid artery occlusions throughout our study period.
Despite a low risk of malignant behaviour, surgical resection remains the treatment of choice for CBT. In our series, we reserved conservative management only for elderly patients with extensive co-morbidities or in those patients with multiple tumours where operative intervention had a high risk of severe debility due to the potential for injury to multiple cranial nerves. Gordon-Taylor (1940) described a meticulous subadventitial “whiteline” dissection which aimed to enucleate the tumour without disturbance of the carotid vessels 2. Early case series reported the necessity of internal carotid artery patency, particularly in patients with CBTs encasing the internal carotid artery and where internal carotid artery occlusion was associated with unacceptably high peri-operative stroke rates of 30% 10, 24. We advocate the insertion of an intraluminal shunt to maintain cerebral perfusion in such patients which may then facilitate the subsequent reconstruction of the resected section of carotid artery using saphenous vein grafts. This was required in 19% of cases in our series where the median tumour size was 5cm in diameter. Netterville et al (1995) reported that vascular repair was performed in only 10% of patients when the tumour was less than 5cm compared to 55.5% in patients with tumours larger than 5cm 25. Therefore the authors feel that a pre-operative diagnosis of tumour size of 5cm or more suggests that a carotid resection and reconstruction is more likely.
Other authors report the ligation of the external carotid artery to devascularise feeding vessels of the tumour10, 15. Temporary external carotid artery occlusion may also be used to minimise bleeding. However, we have never routinely performed elective ligation of the external carotid artery except in four (13%) patients where it was necessary for either haemostasis or to facilitate access to the CBT. External carotid artery ligation varies from 0% to 56% in the reported literature (Table 4).
Cranial nerve damage is the most common early local complication following excision of CBTs due to the close proximity of facial, glossopharyngeal, vagus, accessory and hypoglossal nerves to the CBT itself or secondary to invasion of these nerves by tumour expansion and distortion of normal anatomy by the tumour leading to inadvertent damage during dissection. We report a 27% transient and 30% permanent nerve injury rate for this series. This is comparable to other case series where reports of transient and permanent cranial nerve damage vary from 0% to 54% and 0% to 38% respectively (Table 4). However, it is important to acknowledge that due to the retrospective nature of our study, pre-operative cranial nerve involvement or impairment was rarely recorded in the early patients from our series. Also the inclusion of three GITs correlated with obligatory damage of the vagus nerve in each case.
Cranial nerve injuries identified post-operatively by the operating vascular surgeon were referred on to the appropriate specialty, usually Otolaryngology, for a more detailed assessment and treatment where necessary. The majority of cranial nerve injuries occurred early in the series with a subsequent reduction identified over time with increasing experience.
Post—operative cranial nerve injury is known to correlate with the Shamblin classification of the tumour 7. A higher risk of cranial nerve injury may also be attributed to vascular reconstructive procedures during resection. However, this may just reflect tumour morphology of invasion and higher Shamblin classification 8. Due to the higher number of tumours unclassified at the time of operation we are unable to perform an accurate subgroup analysis on this factor. However it was found in this series that cranial nerve injury was more likely following the removal of larger tumours. The average tumour size in patients who suffered no cranial nerve injury being 3cm while those that suffered cranial nerve injuries was 3.95cm (transient injury = 3.7cm and permanent = 4.3cm).
Specific nerves such as the vagus are more often affected during CBT surgery because of occasional nerve retraction or sacrifice to facilitate tumour excision. In addition, excision of GITs almost always requires vagal nerve sacrifice followed by vocal cord paralysis which was observed in all three patients in our series who had GITs removed. Although not identified in our series, other early local complications may also include bilateral loss of carotid body or sinus function, which can lead to adverse blood pressure control, and bilateral loss of chemoreceptor function which may cause severe hypoventilation necessitating ventilatory support post-operatively 3.
Post-operatively, radiotherapy is indicated where histological analysis demonstrates an infiltrative growth pattern. Local control rates for CBTs between 96% and 100% have been reported with radiotherapy alone. However, this practice should be balanced against the potential risks of subsequent radiation-induced malignant degeneration particularly in younger patients 19-24. Other side effects from radiotherapy may include ageusia, xerostomia and a skin rash. Adjuvant radiotherapy was administered to three patients (10%) in our study because of capsular infiltration, regional lymph node spread and local recurrence respectively. Only the latter patient developed further recurrence and died of metastatic disease. Seven out of the forty case series reviewed documented the use of adjuvant radiotherapy post-CBT excision at rates between 2% and 15% (Table 4).
Clinical follow-up is paramount to identify any evidence of tumour recurrence or development of a contra-lateral CBT especially in patients with a family history or with confirmed underlying gene mutations. Sequential clinical examination is unreliable so we advocate an annual duplex ultrasound with two yearly MRA, which has an increased sensitivity for multicentric tumours in patients with a history of multiple paragangliomas or gene mutations 19. Screening of family members for occult disease should also be considered as an asymptomatic CBT in our series was identified in the father of a patient with bilateral CBTs who was known to be a SDHD gene mutation carrier 5.
CONCLUSION
Management of CBTs remains complex and technically challenging. Despite a dramatic reduction in stroke and mortality, significant morbidity is still associated with surgical treatment particularly to the adjacent cranial nerves. Our long-term experience is comparable with other modern case series reports where surgical intervention carried a low risk of stroke or death and conferred a long-term survival advantage.
KEY LEARNING POINTS.
Over 22 years the incidence of CBTs in Northern Ireland was 0.08/100,000 people/year.
Our experience is comparable with other modern case series reports where surgical intervention carried a low risk of stroke or death.
The management of CBTs requires the ability to safely reconstruct carotid vasculature. This was required in 19% of cases in our series where the median tumour size was 5cm in diameter.
Morbidity from surgery mainly results from the persisting risk of cranial nerve injury.
Conservative management may be considered for those unfit for surgery as the majority of tumours are benign.
REFERENCES
- 1.Sajid MS, Hamilton G, Baker DM. A multicentre review of carotid body tumour management. Eur J Vasc Endovasc Surg. 2007;34(2):127–30. doi: 10.1016/j.ejvs.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Plukker JT, Brongers EP, Vermey A, Krikke A, van den Dungen JJ. Outcome of surgical treatment for carotid body paraganglioma. Br J Surg. 2001;88(10):1382–6. doi: 10.1046/j.0007-1323.2001.01878.x. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Cuevas S., Lopez-Garza J, Labastidia-Almendaro S. Carotid body tumors in inhabitants of altitudes higher than 2000 meters above sea level. Head Neck. 1998;20(5):374–8. doi: 10.1002/(sici)1097-0347(199808)20:5<374::aid-hed3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Luna-Ortiz K, Rascon-Ortiz M, Villavicencio-Valencia V, Granados-Garcia M, Herrera-Gomez A. Carotid body tumours: a review of a 20 year experience. Oral Oncol. 2005;41(1):56–61. doi: 10.1016/j.oraloncology.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Wang SJ, Wang MB, Barauskas TM, Calcaterra TC. Surgical management of carotid body tumors. Otolaryngol Head Neck Surg. 2000;123(3):202–6. doi: 10.1067/mhn.2000.106709. [DOI] [PubMed] [Google Scholar]
- 6.Mathews FS. Surgery of the neck, in Johnson AB (ed): Operative Therapeusis. New York, Appleton-Crofts Inc, 1915, vol 3, chap 9, pg 315. In Farr HW. Carotid Body Tumors: A 40-Year Study. CA Cancer J Clin. 1980;30(5):260–265. doi: 10.3322/canjclin.30.5.260. [DOI] [PubMed] [Google Scholar]
- 7.Shamblin WR, Remine WH, Sheps SG, Harrison EG. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122(6):732–9. doi: 10.1016/0002-9610(71)90436-3. [DOI] [PubMed] [Google Scholar]
- 8.Smith JJ, Passman MA, Dattilo JB, Guzman RJ, Naslund TC, Netterville JL, et al. Carotid body tumor resection: does the need for vascular reconstruction worsen outcome? Ann Vasc Surg. 2006;20(4):435–9. doi: 10.1007/s10016-006-9093-0. [DOI] [PubMed] [Google Scholar]
- 9.Van der Bogt K, Vrancken Peeters M, can Baalen JM, Hamming JF. Resection of carotid body tumors: results of an evolving surgical technique. Ann Surg. 2008;247(5):877–884. doi: 10.1097/SLA.0b013e3181656cc0. [DOI] [PubMed] [Google Scholar]
- 10.Koskas F, Vignes S, Khalil, Koskas I, Dziekiewicz M, Elmkies F, et al. Carotid chemodectomas: long-term results of subadventitial resection with deliberate external carotid resection. Ann Vasc Surg. 2009;23(1):67–75. doi: 10.1016/j.avsg.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Patetsios P, Gable DR, Garrett WV, Lamont JP, Kuhn JA, Shutze WP, et al. Management of carotid body paragangliomas and review of a 30-year experience. Ann Vasc Surg. 2002;16(3):331–8. doi: 10.1007/s10016-001-0106-8. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell RO, Richardson JD, Lambert GE. Characteristics, surgical management, and outcomes in 17 carotid body tumors. Am Surg. 1996;62(12):1034–37. [PubMed] [Google Scholar]
- 13.Davidovic LB, Djukic VB, Vasic DM, Sindjelic RP, Duvnjak SN. Diagnosis and treatment of carotid body paraganglioma: 21 years of experience at a clinical centre of Serbia. World J Surg Oncol. 2005;3(1):10. doi: 10.1186/1477-7819-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pachedo-Ojeda L, Durango E, Rodriguez C, Vivar N. Carotid body tumors at high altitudes: Quito, Ecuador 1987. World J Surg. 1988;12(6):856–60. doi: 10.1007/BF01655498. [DOI] [PubMed] [Google Scholar]
- 15.Persky MS, Setton A, Niimi Y, Hartman J, Frank D, Berenstein A. Combined endovascular and surgical treatment of head and neck paraganglioma — a team approach. Head Neck. 2002;24(5):423–31. doi: 10.1002/hed.10068. [DOI] [PubMed] [Google Scholar]
- 16.Matticari S, Credi G, Pratesi C, Bertini D. Diagnosis and surgical treatment of carotid body tumors. J Cardiovasc Surg (Torino). 1995;36(3):233–9. [PubMed] [Google Scholar]
- 17.Muhm M, Polterauer P, Gsottner W, Temmel A, Richling B, Undt G, et al. Diagnostic and therapeutic approaches to carotid body tumors. Review of 24 patients. Arch Surg. 1997;132(3):279–84. doi: 10.1001/archsurg.1997.01430270065013. [DOI] [PubMed] [Google Scholar]
- 18.Antonitsis P, Saratzis N, Velissaris I, Lazaridis I, Melas N, Ginis G, et al. Management of cervical paragangliomas; review of a 15-year experience. Langenbecks Arch Surg. 2006;391(4):396–402. doi: 10.1007/s00423-006-0047-3. [DOI] [PubMed] [Google Scholar]
- 19.Makeieff M, Raingeard I, Alric P, Bonafe A, Guerrier B, Marty-Ane Ch. Surgical management of carotid body tumors. Ann Surg Oncol. 2008;15(8):2180–6. doi: 10.1245/s10434-008-9977-z. [DOI] [PubMed] [Google Scholar]
- 20.Litle VR, Reilly LM, Ramos TK. Preoperative embolization of carotid body tumors: when is it appropriate? Ann Vasc Surg. 1996;10(5):464–8. doi: 10.1007/BF02000594. [DOI] [PubMed] [Google Scholar]
- 21.LaMuraglia GM, Fabian RL, Brewster DC, Pile-Spellman J, Darling RC, Cambria RP, et al. The current surgical management of carotid paragangliomas. J Vasc Surg. 1992;15(6):1038–45. doi: 10.1067/mva.1992.35505. [DOI] [PubMed] [Google Scholar]
- 22.Wax MK, Briant TD. Carotid body tumors: a review. J Otolaryngol. 1992;21(4):277–85. [PubMed] [Google Scholar]
- 23.Westerband A, Hunter GC, Cintora I, Coulthard SW, Hirni ML, Gentile AT, et al. Current trends in detection and management of carotid body tumors. J Vasc Surg. 1998;28(1):84–92. doi: 10.1016/s0741-5214(98)70203-4. [DOI] [PubMed] [Google Scholar]
- 24.Hallet JW, Nora DJ, Holler LH, Cherry KJ, Pairolero PC. Trends in neurovascular complications of surgical management for carotid body and cervical paragangliomas: a fifty year experience with 153 tumors. J Vasc Surg. 1988;7(2):284–91. [PubMed] [Google Scholar]
- 25.Netterville JL, Reilly KM, Robertson D, Reiber ME, Armstrong WB, Childs P. Carotid body tumours: a review of 30 patients with 46 tumors. Laryngoscope. 1995;105(2):115–26. doi: 10.1288/00005537-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Rosen IB, Palmer JA, Goldberg M, Mustard RA. Vascular problems associated with carotid body tumors. Am J Surg. 1981;142(4):459–63. doi: 10.1016/0002-9610(81)90375-5. [DOI] [PubMed] [Google Scholar]
- 27.Williams MD, Phillips MJ, Nelson WR, Nelson WR, Rainer WG. Carotid body tumor. Arch Surg. 1992;127(8):963–7. doi: 10.1001/archsurg.1992.01420080097016. [DOI] [PubMed] [Google Scholar]
- 28.Papaspyrou K, Mann WJ, Amedee R. Management of head and neck paragangliomas: review of 120 patients. Head Neck. 2009;31(3):381–7. doi: 10.1002/hed.20967. [DOI] [PubMed] [Google Scholar]
- 29.Bakoyiannis KC, Georgopoulos SE, Klonaris CN, Tsekouras NS, Felekouras ES, Pikoulis EA, et al. Surgical treatment of carotid body tumours. Int Angiol. 2006;25(1):40–5. [PubMed] [Google Scholar]
- 30.Kasper GC, Welling RE, Wladis AR, CaJacob DE, Grisham AD, Tomsick TA, et al. A multidisciplinary approach to carotid paragangliomas. Vasc Endovascular Surg. 2006;40(6):467–74. doi: 10.1177/1538574406290254. [DOI] [PubMed] [Google Scholar]
- 31.Knight TT, Gonzalez JA, Rary JM, Rush DS. Current concepts for the surgical management of carotid body tumor. Am J Surg. 2006;191(1):104–10. doi: 10.1016/j.amjsurg.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Dardik A, Eisele DW, Williams GM, Perler BA. A contemporary assessment of carotid body tumor surgery. Vasc Endovascular Surg. 2002;36(4):277–83. doi: 10.1177/153857440203600405. [DOI] [PubMed] [Google Scholar]
- 33.Thabet MH, Kotob H. Cervical paragangliomas: diagnosis, management and complications. J Laryngol Otol. 2001;115(6):467–74. doi: 10.1258/0022215011908180. [DOI] [PubMed] [Google Scholar]
- 34.Liapis CD, Evangelidakis EL, Papavassiliou VG, Kakiskas JD, Gougoulakis AG, Polyzos AK, et al. Role of malignancy and preoperative embolization in the management of carotid body tumors. World J Surg. 2000;24(12):1526–30. doi: 10.1007/s002680010272. [DOI] [PubMed] [Google Scholar]
- 35.Bastounis E, Maltezos C, Pikoulis E, Leppäniemi A, Klonaris C, Papalambros E. Surgical treatment of carotid body tumours. Eur J Surg. 1999;165(3):198–202. doi: 10.1080/110241599750007045. [DOI] [PubMed] [Google Scholar]
- 36.Leonetti JP, Donzelli JJ, Littooy FN, Farrell BP. Perioperative strategies in the management of carotid body tumors. Otolaryngol Head Neck Surg. 1997;117(1):111–5. doi: 10.1016/S0194-59989770216-X. [DOI] [PubMed] [Google Scholar]
- 37.McPherson GA, Halliday AW, Mansfield AO. Carotid body tumours and other cervical paragangliomas: diagnosis and management in 25 patients. Br J Surg. 1989;76(6):33–6. doi: 10.1002/bjs.1800760111. [DOI] [PubMed] [Google Scholar]
- 38.Gaylis H, Davidge-Pitts K, Pantanowitz D. Carotid body tumours. A review of 52 cases. S Afr Med J. 1987;72(7):493–6. [PubMed] [Google Scholar]
- 39.Dickinson PH, Griffin SM, Guy AJ, Mc Neill IF. Carotid body tumour: 30 year experience. Br J Surg. 1986;73(1):14–16. doi: 10.1002/bjs.1800730107. [DOI] [PubMed] [Google Scholar]
- 40.Lees CD, Levine HL, Beven EG, Tucker HM. Tumors of the carotid body. Experience with 41 operative cases. Am J Surg. 1981;142(3):362–5. doi: 10.1016/0002-9610(81)90349-4. [DOI] [PubMed] [Google Scholar]


