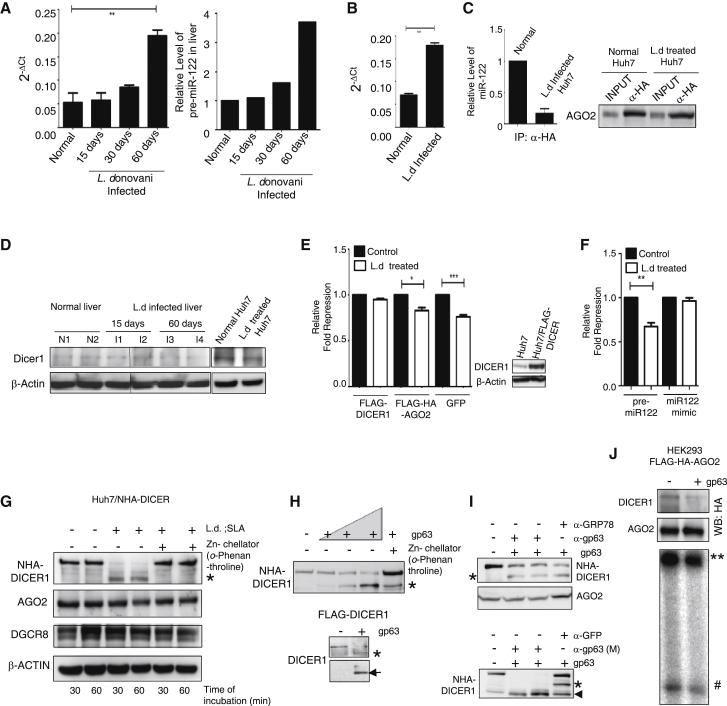
Figure 5.
L. donovani Downregulates DICER1 and Prevents miRNP Formation in Huh7 Cells
(A) L. donovani increases pre-miR-122 levels in livers of L donovani-infected animals. RNA isolated from the livers of BALB/c mice after 15, 30, or 60 days infected were subjected to real-time analysis, and β-actin mRNA levels were used for normalization. n = 6 for each group.
(B) pre-miR-122 levels in Huh7 cells before and after treatment with L. donovani promastigotes for 24 hr. Real-time quantification for pre-miR-122 was performed. SDs were from three independent measurements.
(C) Association of miR-122 with AGO2 in Huh7 cells interacted with L. donovani. RNA was isolated from immunoprecipitated (IP) materials from normal and infected cell lysates, and miR-122 levels were quantified by real-time quantification and normalized against immunoprecipitated AGO2. Quantification data are the mean values obtained from three IP reactions. Western blot was used to detect AGO2 in immunoprecipitated materials.
(D) Dicer1 levels in Leishmania-infected animal livers. Protein extracts were prepared from normal and infected mouse livers (15 or 60 days p.i.) and western blotted for Dicer1. Huh7 cells treated with L. donovani also showed reduction in DICER1 levels.
(E) Exogenous expression of DICER1 inhibits L. donovani-mediated inhibition of miR-122 activity in Huh7 cells. Cells expressing RL reporters were transfected with plasmids encoding FLAG-DICER1, FLAG-HA-AGO2, or GFP, and the effects of L. donovani on miR-122 activity was scored. DICER1 overexpression was confirmed by western blot.
(F) Unlike pmiR-122 overexpressed cells, cells transfected with miR-122 mimics can escape L. donovani-mediated repression of miR-122 activity. miR-122 repressive activity was measured in normal and L.donovani-interacting Huh7 cells expressing pre-miR-122 or miR-122 mimic.
(G) DICER1 can be specifically cleaved by the Zn-metalloprotease present in the SLAs. Huh7 extracts were incubated with SLA in the presence and absence of Zn-chelator for 30 or 60 min at 37°C and were western blotted for HA-DICER1, AGO2, and DGCR8. β-actin was used as loading control.
(H) L. donovani surface protease gp63 cleaves DICER1 and generates two fragments in vitro. Lysates of HEK293T cells expressing NHA-DICER1 were incubated with an increasing concentration of purified gp63 in the absence and presence of Zn-chelator, and cleaved product was visualized by western blot using an HA-specific antibody. It detected a shortened DICER1 band that was ∼180 KDa after cleavage of full-length protein by gp63. Digestion of FLAG-DICER1 also generated a 180 KDa fragment (marked by ∗). The N-terminal and short C-terminal half (marked by an arrowhead) of DICER1 were both detected with a DICER1-specific antibody.
(I) Treatment with specific polyclonal and/or monoclonal antibody modify gp63 activity in vitro. Blocking of gp63 with a polyclonal antibody raised against the recombinant protein reduces DICER1 cleavage activity. Anti-GRP78 antibody was used as control (upper panel). Pretreatment of gp63 against a monoclonal antibody augments its activity in DICER1 cleavage assay (lower panel). Anti-GFP antibody was used as control. The arrowhead denotes a secondary cleaved product increased in the presence of anti-gp63 monoclonal antibody.
(J) The cleavage of AGO2-associated NHA-DICER1 by gp63 and its effect on pre-miRNA processing. Extracts of HEK293 stably expressing FLAG-HA-AGO2 and transfected with NHA-DICER1 were digested with purified gp63 and subsequently FLAG-HA-AGO2 was immunoprecipitated. FLAG-HA-AGO2-associated DICER1 was detected by western blot, and pre-miR-122 processing activities associated with immunoprecipitated materials were quantified. ∗∗ denotes the pre-miR-122 substrate; # denotes the mature miR-122 formed; WB, western blot. Data represent mean ± SEM. See also Figure S4.