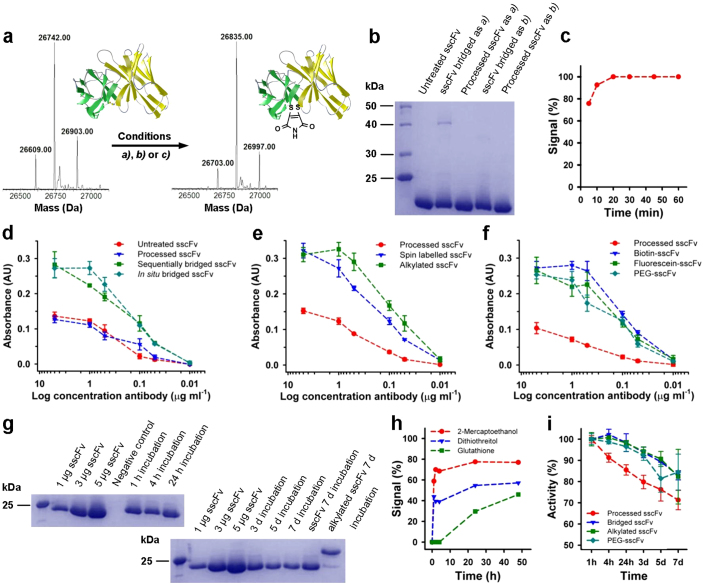
Figure 2. Synthesis, activity and stability of CEA-specific sscFv analogues.
(a) Deconvoluted LC-MS spectra of unmodified and maleimide-bridged sscFv (requires 26,836 Da). Conditions a) (sequential): 20 equiv DTT for 1 h, then 30 equiv of dibromomaleimide; conditions b) (in situ): 15 equiv dithiophenolmaleimide and 15 equiv benzeneselenol; conditions c) (in situ): 2 equiv dithiophenolmaleimide and 25 equiv benzeneselenol. The additional peaks are disulfide bond containing impurities in the starting material. (b) SDS-PAGE of sequentially and in situ bridged sscFv. Processed antibody was treated with maleimides but no reducing agent. (c) Timed LC-MS data of the in situ bridging reaction of sscFv. The signal refers to the relative abundance of the mass ion of bridged sscFv. (d) ELISA of variably bridged sscFv against full length CEA. (e) ELISA of alkylated and functionalized sscFv against full length CEA. (f) ELISA of functionalized sscFv against full length CEA. (g) SDS-PAGE of bridged sscFv isolated from human plasma after incubation at 37°C and loading controls. Unmodified and alkylated sscFv were treated for 7 d and isolated. The negative control contained no antibody. (h) Stability of the bridged sscFv against a 100× excess of thiols as determined by LC-MS. The signal refers to the relative abundance of the mass ion of the un-bridged sscFv. (i) Normalized activity of the sscFv and its analogues after incubation in human plasma at 37°C as determined by ELISA against full length CEA. Full-length gels are presented in Supplementary Figure S16. ELISAs d–f and i were performed in triplicates; error bars show s.d.