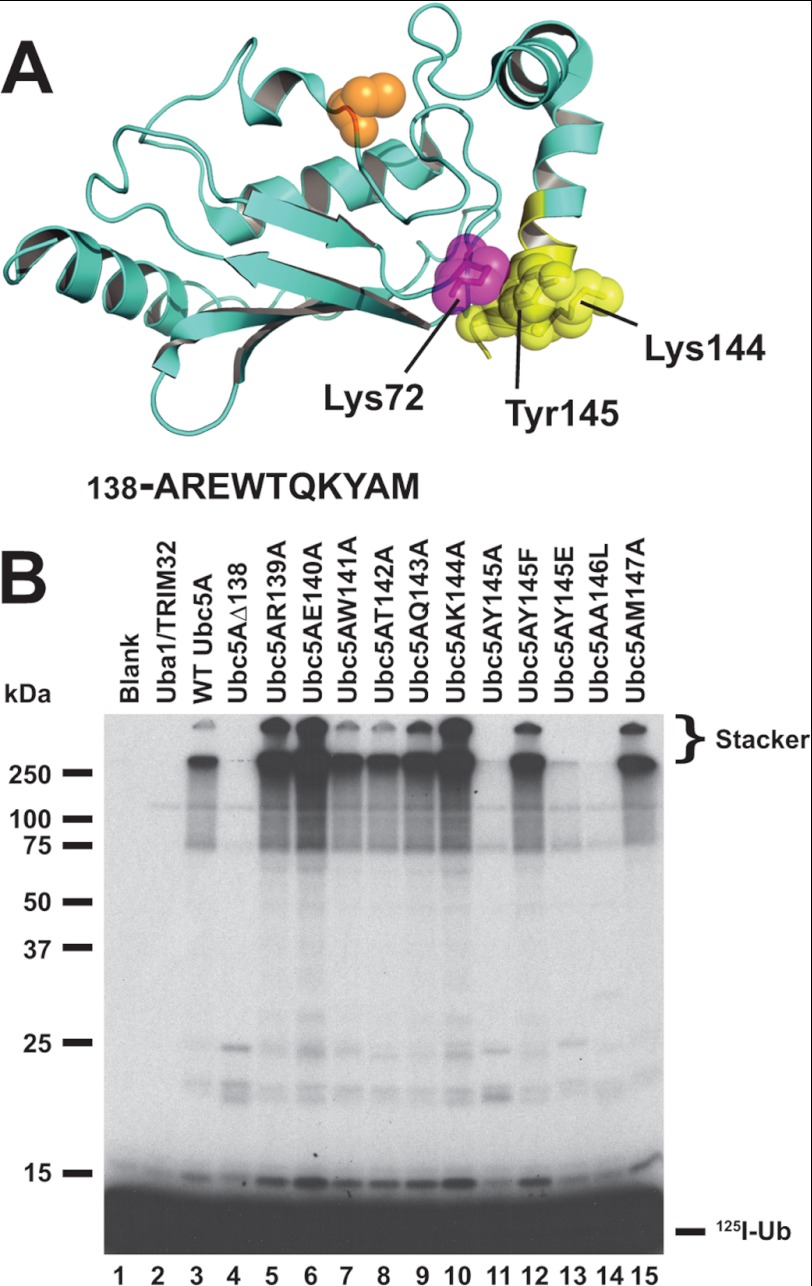
FIGURE 6.
Mutations in the C-terminal α-helix of Ubc5A abrogate polyubiquitin chain formation. A, structure of Ubc5A with the active cysteine shown in orange spheres and the last 10 residues of the protein within the C-terminal α-helix are highlighted in yellow. The packing of Tyr145 between Lys72 and Lys146 is highlighted. The sequence for the C-terminal 10 residues is shown below the structure. The image was created from PDB file 2C4P (Dodd, R. B., and Read, R. J., unpublished coordinates) using PyMol (Schrodinger). B, autoradiogram comparing wild type Ubc5A (lane 3) and the indicated C-terminal point mutants (lanes 4–15) in their ability to support TRIM32-catalyzed 125I-ubiquitin chain formation. Reactions contained 64 nm Uba1, 100 nm active E2, and 200 nm TRIM32 protein, as indicated. Migration positions for relative molecular weight standards are shown to the left. The position of the 5% stacking gel and free 125I-ubiquitin are shown to the right.