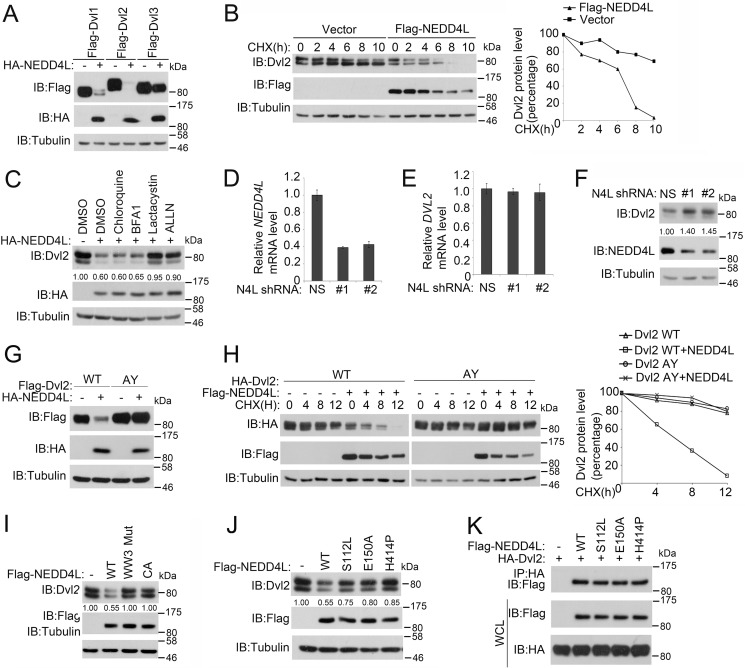
FIGURE 3.
NEDD4L promotes Dvl2 degradation. A, NEDD4L induces degradation of three Dvl members. After transfection with human FLAG-Dvl1, -2, and -3 together with or without HA-NEDD4L for 48 h, HEK293T cells were harvested for immunoblotting (IB). Tubulin served as a loading control. B, NEDD4L decreases Dvl2 half-life. After HEK293T cells were transfected with FLAG-NEDD4L for 36 h, cells were treated with cycloheximide (CHX) (20 μg/ml) for the indicated times and harvested for immunoblotting. Tubulin served as a loading control. Dvl2 levels were quantified. C, NEDD4L degrades Dvl2 through the ubiquitin-proteasome pathway. HEK293T cells transfected with HA-NEDD4L were treated with lysosome inhibitors bafilomycin A1 (0.2 μm) and chloroquine (50 μm) or proteasome inhibitors lactacystin (10 μm) and ALLN (30 μm) for 4 h before harvesting for immunoblotting. Tubulin served as a loading control. The lower panel is the quantitation of Dvl2 band density. D and E, NEDD4L knockdown has no effect on the Dvl2 mRNA level. After HEK293T cells were transfected with nonspecific (NS) or two independent NEDD4L shRNAs for 72 h, total RNA was extracted and subjected to quantitative RT-PCR for examine NEDD4L (D) or Dvl2 (E) mRNA expression. The results show mean ± S.D. (n = 3). F, NEDD4L knockdown increases the Dvl2 level. After HEK293T cells were transfected with NS or two independent NEDD4L shRNAs for 72 h, cells were harvested for immunoblotting. Tubulin served as a loading control. G, the PY motif of Dvl2 is necessary for NEDD4L-induced degradation. After wild-type or AY mutant FLAG-Dvl2 was transfected with or without HA-NEDD4L into HEK293T cells for 48 h, cells were harvested for immunoblotting. Tubulin served as a loading control. H, NEDD4L decreases the half-life of wild-type Dvl2, but not Dvl2 (AY). The experiment was performed similarly as in B. I, the WW3 domain and ubiquitin ligase activity of NEDD4L are required for its induction of Dvl2 degradation. After wild-type or WW3 mutant or CA FLAG-NEDD4L was transfected into HEK293T cells for 48 h, cells were harvested for immunoblotting. Tubulin served as a loading control. J, point mutations of NEDD4L associated with epilepsy attenuate its ability to degrade Dvl2. The experiment was preformed similarly as in I. K, mutations of NEDD4L associated with epilepsy do not affect the interaction with Dvl2. HEK293T cells were transfected with the indicated constructs. After 36 h, the cells were treated with 30 μm ALLN for 4 h before harvesting for anti-HA immunoprecipitation (IP) followed by anti-FLAG immunoblotting. Total protein expression was confirmed by immunoblotting with whole cell lysates (WCL) (lower panels).