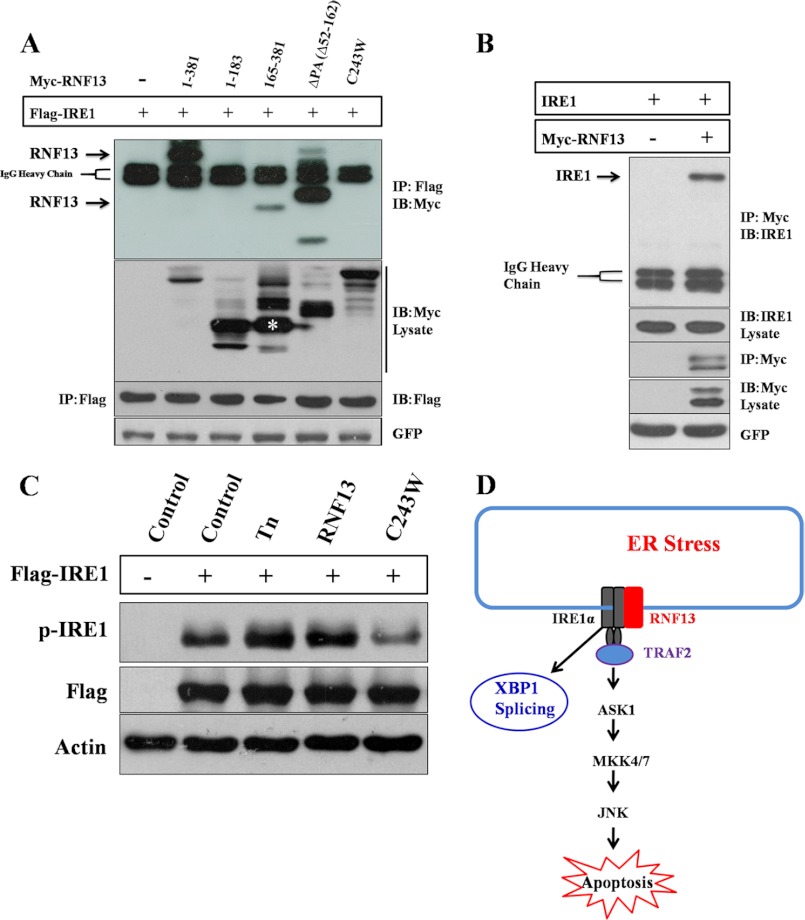
FIGURE 7.
RNF13 interacts with IRE1α and promotes IRE1α phosphorylation. A, 293T cells were co-transfected with 2 μg of FLAG-IRE1α plasmid and 3 μg of plasmid encoding Myc-RNF13 or its mutants for 24 h. FLAG-IRE1α protein was immune-precipitated (IP) using anti-FLAG antibody, and the presence of RNF13 or its mutant proteins in the immunoprecipitated products was detected by anti-Myc antibody. IB, immunoblot. B, RNF13 interacts with endogenous IRE1α. 293T cells were transfected with 3 μg Myc-RNF13 plasmid for 24 h. Myc-RNF13 protein was immunoprecipitated using anti-Myc antibody, and the presence of endogenous IRE1α in the immunoprecipitated products was detected by anti-IRE1α antibody. C, 293T cells were transfected with 2 μg of IRE1α plasmid alone or co-transfected with 3 μg of plasmid encoding RNF13 or its RING domain mutant (C243W) for 24 h. For cells treated with Tn, 1.25 μg/ml drug was added at 18 h post-transfection. D, a schematic model illustrates the role of RNF13 in mediating apoptosis through the IRE1α-TRAF2-JNK signaling pathway.