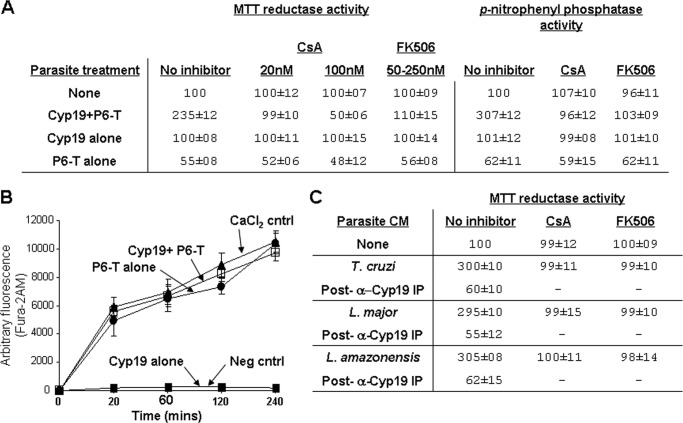
FIGURE 4.
Cyclophilin 19 induces parasite calcineurin phosphatase activation that is dependent on the proline residues within trialysin. A, MTT reductase activity is inhibited by calcineurin inhibitors CsA and FK506. This correlates with the induction of p-nitrophenyl phosphate phosphatase activity in parasite extracts that is also susceptible to inhibition with both inhibitors. B, analysis of intracellular Ca2+ flux induced using Fura-2AM-loaded epimastigotes indicates that Cyp19+P6-trialysin and P6-trialysin alone cause the same kinetics of intracellular Ca2+ increase, whereas Cyp19 treatment does not. Assays were done in non-conditioned M199. For the positive control, medium was supplemented with 10 mm CaCl2. Assays done in PBS in place of M199 showed similar results. Assays were done over 6 h; no significant increase was observed after 4 h. The mean values of arbitrary fluorescence ± S.D. of assays done in triplicate are shown. C, CM from T. cruzi, L. major, and L. amazonensis (as indicated) was used for reductase assays of T. cruzi using WT P6-trialysin. Cyclophilin 19 immunoprecipitation of each medium depleted the reductase activation of parasites and provided a control for Cyp19 specificity. Preimmune serum (PI) had no effect on the ATP content of activated parasites, which correlated with the reductase activity similar to that shown in Fig. 1. CsA (10 nm) and FK506 (250 nm) preincubation of parasites for 30 min inhibits parasite reductase activity induced by all CM. CsA and FK506 alone is not toxic to parasites (as indicated). Dashes indicate that reactions were not done. MTT reductase activity is expressed as the mean % of untreated controls ± S.D. (n = 9 in panels A and C, n = 6 in panel B).