FIGURE 5.
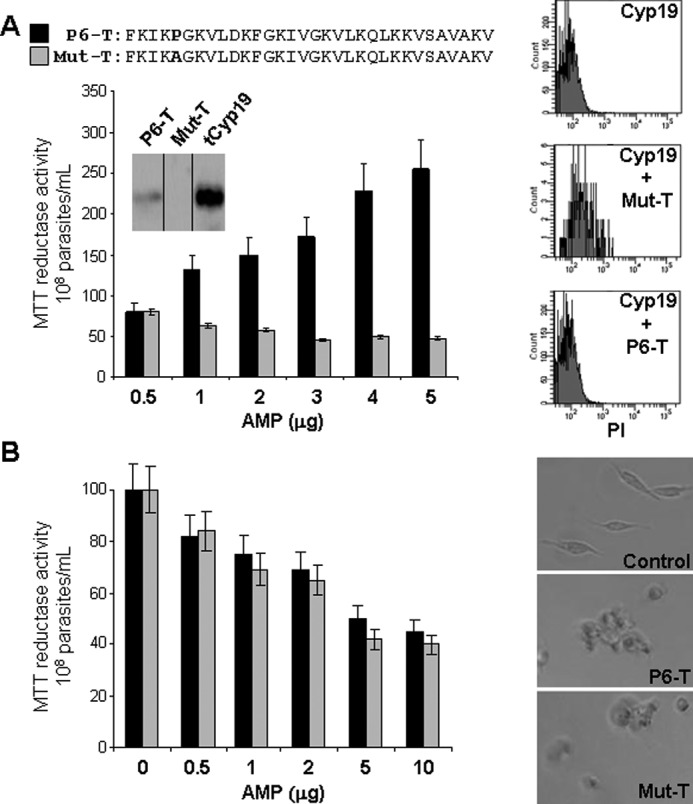
Cyclophilin evasion of antimicrobial peptide killing and bioenergetic activation is dependent on the proline residue within trialysin. A, left panel, a wild type and the proline replacement mutant of P6-trialysin and Mut-T (black and gray bars, respectively, as indicated); a comparison for parasite reductase activity shows that the presence of proline is crucial for induction of Cyp19-mediated parasite reductase activity and survival. Inset, anti-trypanosomal Cyp19 Western blots of recombinant trypanosomal Cyp19 pulldown reactions of Dynabeads containing either WT-P6-trialysin or Mut-P6-trialysin (Mut-T). The results indicate that only the WT-P6-trialysin, but not the mutant, is able to bind Cyp19 protein. Right panel, propidium iodide (PI) flow cytometric viability assay of parasites treated with P6-T or Mut-T in the presence of Cyp19 (as indicated) shows that only parasites treated with the Mut-T but not P6-T leads to decreased viability. B, Mut-T and P6-trialysin are equally trypanocidal in non-conditioned medium. Treatment of stationary phase epimastigotes (107 in 100 μl of fresh M199 for 2 h) with the indicated amounts of either P6-T or Mut-T analyzed by MTT reductase activity (left panel) or light microscopy (right panel). Both peptides cause parasites to lose their trypanosome morphology and clump under non-Cyp19-containing conditions. Reductase activity is expressed as % MTT reductase activity of CAMP-untreated (0 peptide control) parasites ± S.D., in M199 which was 100%.
