Abstract
A five-component catalyst assembly/aziridination reaction is described starting from an aldehyde, an amine, ethyl diazoacetate, B(OPh)3 and a molecule of a vaulted biaryl ligand (VAPOL or VANOL). A remarkable level of chemo-selectivity was observed since while 10 different products could have resulted from various reactions between the five components, an aziridine was formed in 85% yield and 98% ee and only two other products could be detected in 3% yield. Studies reveal that the first in a sequence of three reactions is an exceedingly rapid amine induced assembly of an amino-boroxinate (AMINO-BOROX) species from VAPOL and B(OPh)3 which is followed by imine formation from the amine and aldehyde and the concomitant formation of an IMINO-BOROX species, and finally the reaction of the imine with ethyl diazoacetate mediated by the IMINO-BOROX catalyst to give aziridine-2-carboxylic esters with very high diastereo- and enantioselectivity.
1. INTRODUCTION
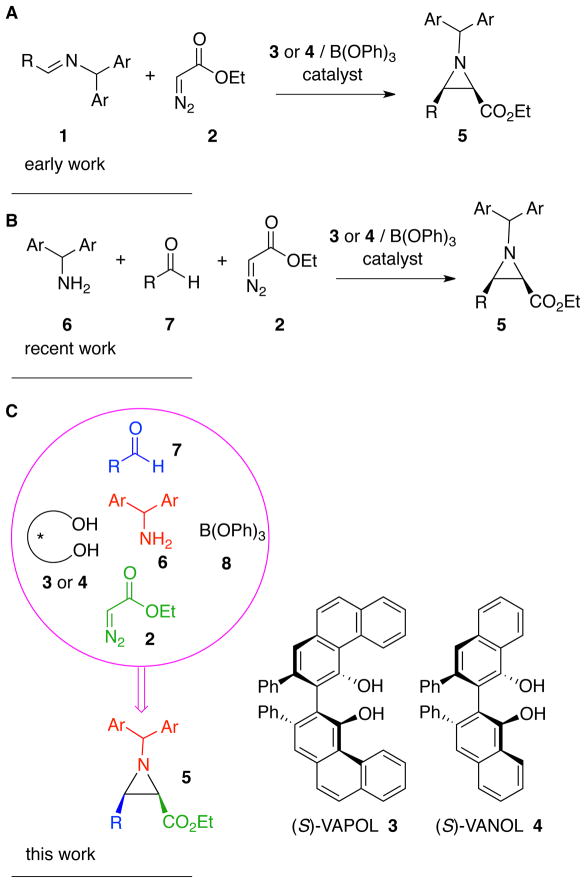
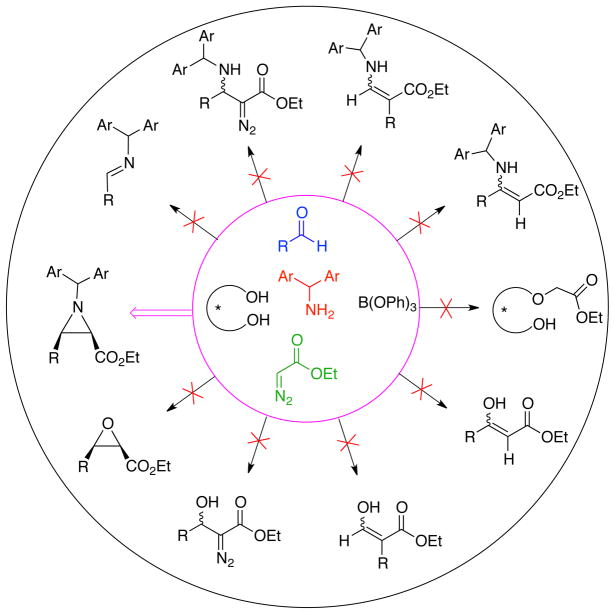
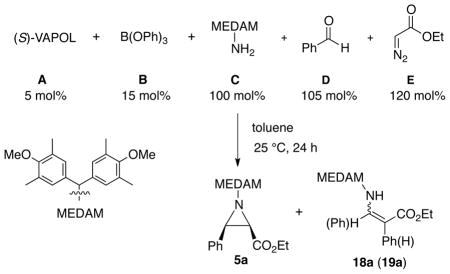
Over the last decade we have developed a catalytic asymmetric aziridination of imines with diazo compounds (AZ reaction) with a chiral pre-catalyst prepared from either of the vaulted biaryl ligands VAPOL 3 or VANOL 4 and B(OPh)3 (Figure 1A).1–4 This AZ reaction is highly enantioselective and diastereoselective giving either cis-1,2 or trans-aziridines2,3 with a variety of imines and diazo compounds. Shortcomings of the optimized protocol include the need to generate a pre-catalyst from the VAPOL or VANOL ligand and B(OPh)3 and the need to prepare an imine from an aldehyde and an amine. The need to prepare the imine has limited the scope of the AZ reaction since many types of aliphatic aldehydes do not form stable imines. We recently reported a solution to these limitations with the development of a three-component catalytic asymmetric aziridination that starts from an amine, an aldehyde and a diazo compound (Figure 1B).5 In the course of studying factors that affect catalyst formation and in investigating the processes involved in the three-component catalytic asymmetric aziridination, and in examining the relationship between these two processes, we have discovered that this reaction is actually a five-component process in which the catalyst is assembled by the amine from the ligand and B(OPh)3 and then the catalyst causes imine formation and then finally, aziridine formation (Figure 1C).6 The possible combinations of all of the outcomes of all the different known reactions between the five species shown in Figure 1C would lead to a mixture of 10 different products and yet a solution of these five-species leads to the formation of the aziridine in very high yields and enantioselectivities. The purpose of the present work is to provide an insight to and support for the operation of this five-component catalyst assembly/catalytic asymmetric aziridination.
Figure 1.
A: catalytic asymmetric aziridination of imines. B: three- component catalytic asymmetric aziridination. C: five- component catalyst assembly/catalytic asymmetric aziridination.
2. RESULTS AND DISCUSSION
Base induced formation of BOROX catalysts from VAPOL and B(OPh)3
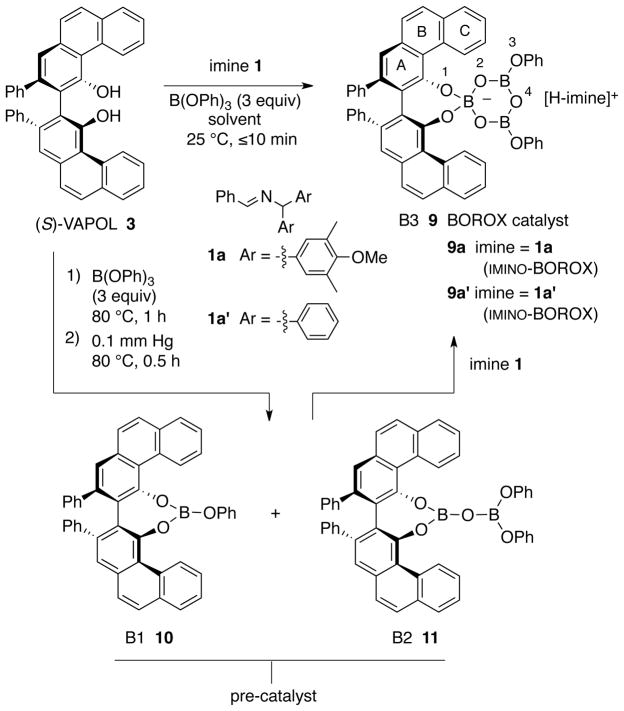
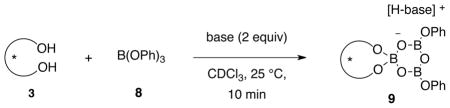
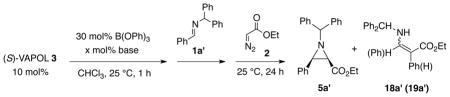
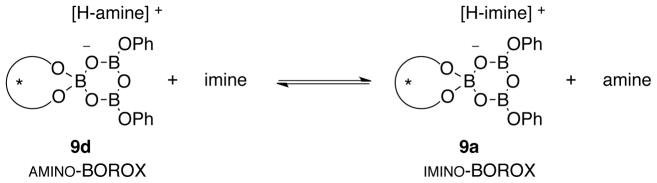
The VAPOL ligand and commercial B(OPh)3 will react when heated to give a pre-catalyst which is a mixture of the mesoborate 10 (B1) and the pyroborate 11 (B2) with the latter typically predominating in ratios ranging from 4:1 to 20:1 depending on the exact protocol for catalyst formation (Scheme 1).1a Treatment of the mixture of 10 and 11 with imine 1 at room temperature leads to the formation of the chiral boroxinate 9 (B3).3b,7 Since this species has a boroxine ring as its core, we have decided to classify this family of catalysts as BOROX catalysts. The BOROX species 9 is an ion pair consisting of a protonated base (imine) as the cation and a chiral polyborate as the anion in the form of a boroxinate: a boroxine in which one of the borons is four-coordinate. It has been shown that the active catalyst in the catalytic aziridination reaction3b,7 is an IMINO-BOROX catalyst of the type 9. This BOROX species is presumed to be the active catalyst in an aza-Diels-Alder reaction as well.8 We have recently reported that the chiral boroxinate 9a (B3) can also be generated upon treatment of a mixture of the VAPOL ligand 3 and 3 equivalents of B(OPh)3 with the imine 1a at room temperature in a few minutes, conditions under which VAPOL and B(OPh)3 do not react.7a The question then naturally arises: what other species can induce the formation of the chiral boroxinate complex 9?9
Scheme 1.
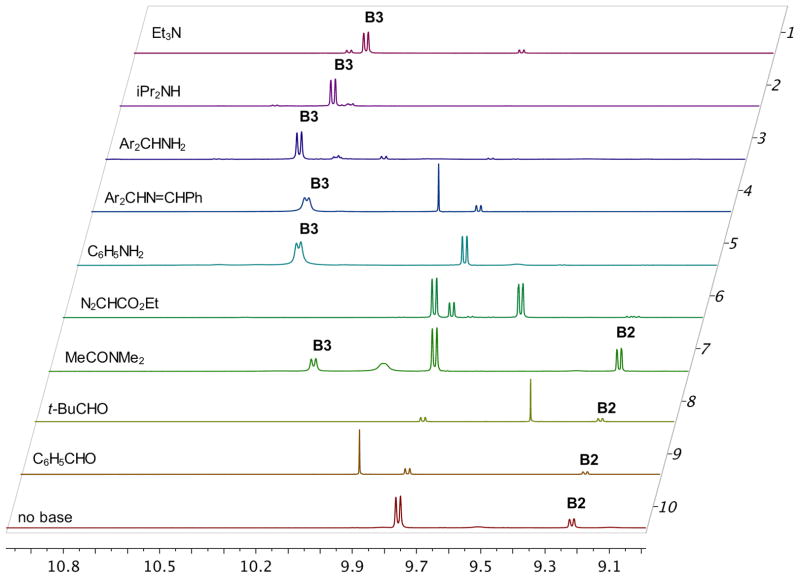
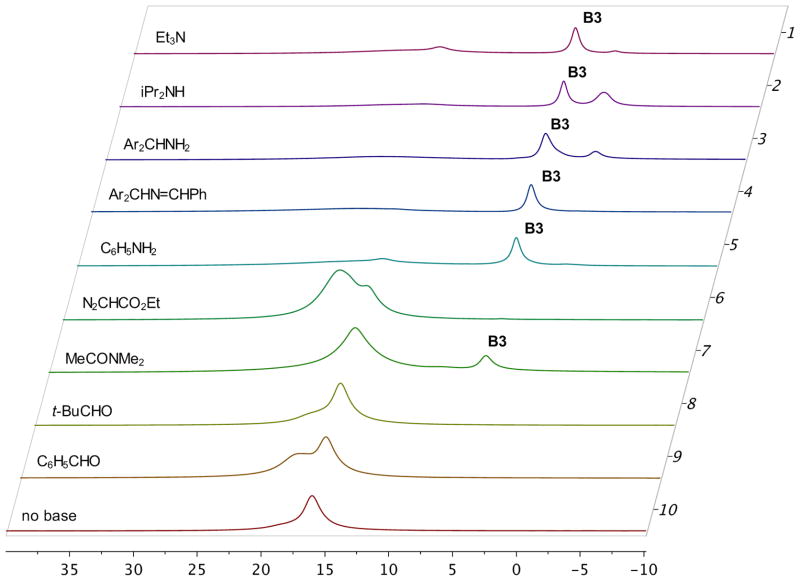
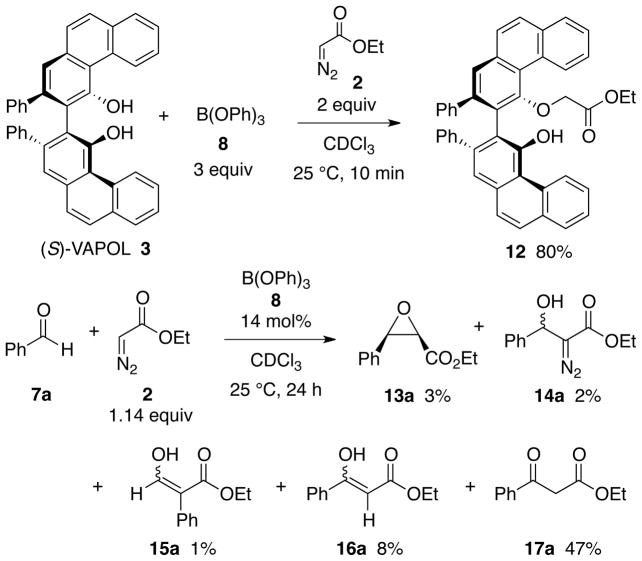
A series of NMR experiments were carried out to probe the effectiveness of various bases to induce the formation of the boroxinate catalyst 9 (B3) and the data are presented in Table 1 and in Figures 2 and 3. In the absence of a base, mixing (S)-VAPOL and B(OPh)3 does not result in any detectable amount of a boroxinate (B3) species (entry 10, Table 1 and Figures 2 & 3). The 1H NMR spectrum of the bay region indicates the presence of unreacted VAPOL (δ 9.77 ppm) and a very small amount (5%) of the mesoborate 10 (B1) (δ 9.55 ppm) and a slightly larger amount (18%) of the pyroborate 11 (B2) (δ 9.22 ppm).10 Primary, secondary and tertiary amines all induce the formation of an AMINO-BOROX catalyst 9 (B3) which displays a characteristic bay proton doublet at δ ~10.3 ppm and a sharp resonance in the 11B NMR spectrum at δ ~ 5–6 ppm. While the four coordinate boron in boroxinate 9 is a relatively sharp absorption (δ~5–6), the two three coordinate borons in 9 have broad resonances at δ = 16–19 ppm which is the region expected for all three coordinate borate esters. The broadness of the absorption at δ = 16–19 ppm makes accurate integration difficult, and in addition, the expansion level shown in Figure 3 makes them difficult to discern. At a greater expansion, the absorptions at δ = 16–19 ppm for the first five entries in Figure 3 are clearly visible (See Supporting Information). Weaker bases such as aniline are also strong enough to induce boroxinate formation but the cut off in basicity seems to be near that of dimethyl acetamide (pKb = −0.5) which generates the boroxinate 9g in only 18% yield. Aldehydes are not strong enough bases to induce the formation of a boroxinate. Ethyl diazoacetate (EDA) does not induce the formation of a boroxinate, but, nonetheless there is a very rapid reaction (≤10 min) leading to the alkylation of one of the phenol functions of VAPOL via a formal insertion of a carbene into the O-H bond which results in the formation of the ester 12 in 80% yield (Table 1, entry 6 and Scheme 2).11
Figure 2.
1H NMR spectra of the bay region of VAPOL boroxinate 9 with the bases in Table 1.
Figure 3.
11B NMR spectra of the VAPOL boroxinate catalyst 9 with the bases in Table 1.
Table 1.
Base Induced Boroxinate Formation from (S)-VAPOL and B(OPh)3.a
| |||||||
|---|---|---|---|---|---|---|---|
| entry | base | (S)-VAPOL 3 (% unreacted)b | % yield 10 (B1)c | % yield 11 (B2)d | % yield 9 (B3) | 9 | 9 (B3) δ (ppm) (1H; 11B)e |
| 1 | Et3N | 8 | <1 | <1 | 63 | 9b | 10.25, 5.37 |
| 2 | i-Pr2NH | <1 | <1 | <1 | 74 | 9c | 10.31, 5.18 |
| 3 | Ar2CHNH2 f | 3 | <1 | <1 | 50 | 9d | 10.37, 5.48 |
| 4 | Ar2CHN=CHPh f,g | 11 | <1 | <1 | 76 | 9a | 10.31, 5.51 |
| 5 | C6H5NH2 | 24 | <1 | <1 | 61 | 9e | 10.29, 5.56 |
| 6 | N2CHCO2Et h | 15 | <1 | <1 | <1 | 9f | – – – – |
| 7 | MeCONMe2 | 32 | <1 | 24 | 18 | 9g | 10.15, 5.78 |
| 8 | t-BuCHO | 37 | <1 | 33 | <1 | 9h | – – – – |
| 9 | C6H5CHO | 52 | <1 | 27 | <1 | 9i | – – – – |
| 10 | no base | 57 | 5 | 18 | <1 | 9j | – – – – |
0.20 mmol of base where added to a solution of 0.10 mmol of (S)-VAPOL and 0.30 mmol of commercial B(OPh)3 in 1.0 mL of CDCl3 at 25 °C and allowed to stir 10 min before spectra were taken. All yields are 1H NMR yields with Ph3CH as internal standard.
Bay region doublet at δ = 9.77 ppm.
Bay region doublet at δ = 9.55 ppm.
Bay region doublet at δ = 9.22 ppm.
Chemical shifts for bay region doublet in 1H NMR and four-coordinate boron in 11B NMR.
Reference 6a.
This reaction produces an 80% yield of the mono-alkylation product 12 (Scheme 2).
Scheme 2.
Predicted structures of IMINO-BOROX species 9a and AMINO-BOROX species 9d by DFT calculations
We have previously reported the solid-state structure of the imino-BOROX catalyst-substrate complex 9a.7a The X-ray analysis revealed the presence of a number of non-covalent interactions between the protonated substrate as it is bound to the anionic boroxinate catalyst. The protonated amine 6 should have a very different binding mode in the boroxinate species 9d due to the absence of the benzylidene group on the nitrogen atom and the presence of an ammonium ion with three hydrogens capable of H-bonding to the anionic boroxinate platform. Unfortunately, the boroxinate complex 9d could not be induced to form single crystals suitable for X–ray analysis.
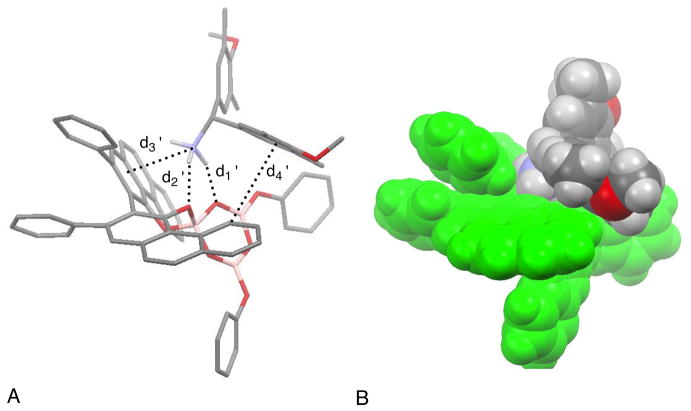
Therefore, computational methods were undertaken to explore the possible structures of the AMINO-BOROX 9d. Calculations were preformed using Gaussian 03 at B3LYP/6-31g* level of theory.12 As a prelude, computations were first performed on the IMINO-BOROX 9a since the results could be compared with the solid state structure determined by X-ray analysis.7a The results of DFT calculations on 9a are shown in Figure 4 and are very similar to the structure determined by X-ray. There are some differences as one might expect for a substrate-catalyst complex in the gas phase versus solid state. The strongest non-covalent interaction would be expected to be the H-bond between the protonated iminium and the boroxinate core. As in the solid state this was found to be with oxygen-2 of the boroxinate core with a calculated distance of 1.73 Å vs. 2.02 Å in the solid state (d1 in Figure 4). Common to both structures is the edge-face interaction (CH-π) interaction between of one of the aryl rings of the MEDAM group (see Table 2) with one of the phenyl rings of the VAPOL ligand (d2 in Figure 4).13 This distance in the calculated structure is 3.92 Å and it is 4.42 Å in the solid state. It should be noted that the CH-π interaction distances are by convention indicted as the distance between the carbon bearing the hydrogen and the centroid of the benzene ring participating in the interaction.14 There are also differences in the orientation of the phenyl group of the benzaldehyde imine relative to one of the phenanthrene walls of the VAPOL ligands (d3 in Figure 4). The contact distance is about the same (3.56 vs 3.44 Å) but the angle between the planes containing these two rings increases from 4 to 39° in the calculated structure. This twisting of the phenyl ring in the calculated structure allows for a closer edge-face interaction between one of the phenoxy groups of the boroxinate core and the phenyl group of the benzaldehyde imine (d4, Figure 4). This distance is 4.66 Å in the calculated structure and 5.52 Å in the solid state. This phenoxy group of the boroxinate core in the calculated structure also moves about a half an Angstrom closer for a better CH-p interaction with one of the methyl groups on the back-end aryl substituent of the MEDAM group (d5 in Figure 4).
Figure 4. Calculated structure of IMINO-BOROX 9a.
(A) Calculated B3LYP/6-31g* structure visualized by the Mercury Program (C, gray; O, red; N, blue; B, pink). Hydrogen atoms omitted for clarity (except N-H). Some secondary interactions are highlighted: d1 = 1.73 Å (H-bonding), d2 = 3.92 Å (CH-p), d3 = 3.56 Å (CH-p or p-p stack), d4 = 4.66 Å (CH-p), d5 = 4.02 Å (CH-p). (B) Space-filling rendition of 9a with the same orientation and showing hydrogens. The boroxinate anion is given in green and the iminium cation is in traditional colors.
Table 2.
Protocol Dependence of the Multi-Component Aziridination.a
| ||||
|---|---|---|---|---|
| procedure | reaction protocol | % yield 5a b | % ee5a c | |
| I | A + B + C |
|
92 | 95 |
| IIA | A + B + C |
|
97 | 98 |
| IIB | A + B |
|
98 | 98 |
| IIIA | A + B + C |
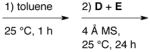
|
98 | 98 |
| IIIB | A + B |
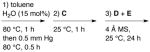
|
94 | 98 |
| IIIC | A + B + C |
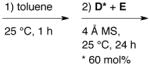
|
<1 | – |
All reactions were performed with 0.025 mmol (S)-VAPOL A (5 mol%), 0.075 mmol B(OPh)3 B (15 mol%), 0.50 mmol amine C (1.0 equiv), 0.525 mmol benzaldehyde D (1.05 equiv) and 0.60 mmol of EDA E (1.2 equiv) in 1.0 mL toluene at 25 °C for 24 h. In each case, 150 mg powdered 4 Å MS was added immediately preceding benzaldehyde.
Isolated yield after chromatography on silica gel. Analysis of the crude reaction mixture indicates ≥50:1 cis:trans selectivity for 5a. In each case <1% 18a and 19a are formed.
Determined on purified cis-5a by HPLC.
The calculated structure for the BOROX species 9d is presented in Figure 5. The key interactions between the cationic protonated amine and the anionic boroxinate core are the two hydrogen bonds from the ammonium ion to O-1 and O-2 of the boroxinated anion (see Scheme 1 for boroxinate numbering). The hydrogen bond to O-2 (d1’ = 1.75 Å) is slightly shorter than that to O-1 (d2’ = 1.96 Å) consistent with the computational finding that the electron density of the boroxinate core is highest at O-2 (see supporting information). A similar computational finding has been previously reported for the VANOL boroxinate core.3b,7b There is also a short distance between the ammonium nitrogen and the centroid of the A-ring of one of the phenanthrene units in the VAPOL ligand (d3’ = 3.21 Å) which is indicative of a NH-π interaction. The iminium ion in 9a and the ammonium ion in 9d both have the protonated nitrogen H-bonded to O-2 of the boroxinate core, but the head groups of the nitrogen substituent (MEDAM group) are on opposite sides of the boroxinate platform. In 9a the MEDAM group has an edge-face interaction with the phenyl substitutent of VAPOL and in 9d, the MEDAM group has an edge-face interaction with the C-ring of VAPOL (d4’ in Figure 5). Both complexes were also examined with BHandHLYP/6-31g* level of theory and the results were not significantly different from that with B3LYP/6-31g*.15
Figure 5. Calculated structure of AMINO-BOROX 9d.
(A) Calculated B3LYP/6-31g* structure visualized by the Mercury Program (C, gray; O, red; N, blue; B, pink). Hydrogen atoms omitted for clarity (except N-H). Some secondary interactions are highlighted: d1’ = 1.75 Å (H-bonding), d2’ = 1.96 Å (H-bonding), d3’ = 3.21 Å (CH-p or cation-p), d4’ = 4.18 Å (CH-π). (B) Space-filling rendition of 9d with the same orientation and showing hydrogens. The boroxinate anion is given in green and the ammonium cation is in traditional colors.
Possible complications for the five-component catalyst assembly/aziridination
As discussed above, the mono-alkylated VAPOL derivative 12 is formed upon treatment of VAPOL with EDA in the presence of B(OPh)3 (Table 1, entry 6). However, there is no reaction between VAPOL and EDA in the absence of B(OPh)3. Nonetheless, this presents at least one potential problem in a five-component catalyst assembly/aziridination of the type shown in Figure 1C. The VAPOL ligand could be disenabled by a B(OPh)3 induced reaction with EDA before amine can assemble the catalyst from the ligand and B(OPh)3. A second possible complication is an unwanted reaction between the aldehyde and EDA. In fact, several different products are known from the reaction of benzaldehyde and EDA mediated by Lewis or Brønsted acids.16 Indeed, in a control experiment, the reaction of benzaldehyde and 1.14 equiv of ethyl diazoacetate mediated by 14 mol% B(OPh)3 gave the five products shown in Scheme 2 with the major component as the β-keto ester 17a and its tautomer 16a in a total of 55% yield. In a separate control experiment it was found that there is no reaction between benzaldehyde and EDA in the presence of VAPOL. Nor is there any reaction between benzaldehyde and EDA in either the presence or absence of 4 Å molecular sieves at 25 °C after 24 h. Thus the possible competing reaction between the aldehyde and EDA mediated by B(OPh)3 is a real concern for the success of the multi-component catalyst assembly/aziridination outlined in Figure 1C.
Different protocols for the multi-component aziridination
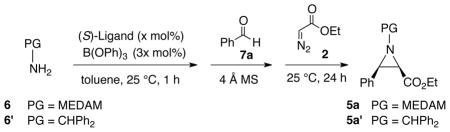
With the demonstration that an amine can induce catalyst assembly, a study of the effects of variation in the conditions for catalyst assembly on the multi-component aziridination (MCAZ) was then explored (Table 2). In the first experiment, the VAPOL ligand A (3) was mixed with 3 equiv of B(OPh)3 B (8) and 20 equiv of amine C (6) and stirred in toluene at 80 °C for 0.5 h to ensure formation of the boroxinate catalyst and thus avoid any possibility of alkylation of VAPOL by the EDA. This was followed by the addition of the benzaldehyde D (7a) and 4 Å MS which was stirred for 2 h to ensure imine formation. Upon addition of EDA E (2) the mixture was stirred at 25 °C for 24 h to give the aziridine 5a in 92% yield and 95% ee (procedure I).
A true multi-component reaction involves the reaction of three or more reagents added simultaneously.17 Multi-component reactions that involve reaction between two reagents and then interception of the resulting intermediate by the addition of a third reagent are sequential component reactions.17–20 Hence, procedure I in Table 2 can be viewed as an example of a sequential component aziridination reaction as the addition of EDA was delayed for 2 h to ensure formation of the imine.19 However, when EDA was added immediately after the aldehyde, essentially the same results were obtained (procedures I vs IIA). This suggests that the present work is an example of a true MCAZ and this will be further verified by the data associated with Table 3. The data in Table 2 reveals that essentially the same results are obtained whether the pre-catalyst is first generated and employed in the multi-component aziridination or whether the boroxinate catalyst is directly generated by treating a mixture of VAPOL and B(OPh)3 with the amine C (procedures IIA vs. IIB). It was also revealed that no advantage is to be gained by generating the AMINO-BOROX catalyst by heating to 80 °C rather than simply stirring at room temperature (procedures IIA vs. IIIA and IIB vs. IIIB). The use of the aldehyde D as the limiting reagent (0.6 equiv) leads to the complete shutdown of the reaction (procedure IIIC). This suggests that the amine C is a competitive inhibitor of the imine in binding to the boroxinate catalyst and this issue will be taken up in greater detail below (Tables 5 and 6). Water is needed for catalyst formation and its addition in procedures IIB and IIIB was done to compensate for any lack of water present in commercial B(OPh)3.7a
Table 3.
Effects of Order of Addition on the Multi-Component Aziridination.a
| procedure | reaction protocol | % yield 5a b | % ee 5a c | |
|---|---|---|---|---|
| IVd | A + B + C + E |
|
94 | 98 |
| Ve |
A + B + D* + E + C * plus 4 Å MS |
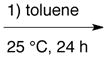
|
85 f | 98 |
| VIg |
A + B + D* + E * plus 4 Å MS |
|
<1 | – |
| VIIh |
A + B + D* + E *plus 4 Å MS |
|
– | – |
All reactions were performed as described in Table 2.
Isolated yield after chromatography on silica gel. Analysis of the crude reaction mixture indicates =50:1 cis:trans selectivity for 5a.
Determined on purified cis-5a by HPLC.
The diazo compound E is added to a mixture of A, B and C and then toluene is added and the mixture stirred at 25 °C for 1 h after which 4 Å powdered molecular sieves and D are added and the mixture stirred for 24 h at 25 °C.
A mixture of A, B, 4 Å MS, D, E and C, added in that order, was dissolved in toluene and the resulting solution allowed to stir for 24 h at 25 °C.
A 3% yield of enamines 18a and 19a was observed.
A mixture of A, B, 4 Å MS, D and E, added in that order, was dissolved in toluene and the resulting solution allowed to stir for 2 min at 25 °C before C was added and the resulting solution stirred for 24 h at 25 °C. The products of this reaction are shown in Figure 6.
Same as procedure VI except that C was not added. The products from this reaction are 13a (5%), 14a (<1%), 15a (5%), 16a (11%), 17a (38%) and 12 (37%).
Table 5.
Multi-component aziridination with a deficiency of benzaldehyde.a

| ||||||
|---|---|---|---|---|---|---|
| entry | 7a x equiv | % yield cis-5a'b,c | % eed cis-5a'd | cis/transb | % yield 18a' + 19a'b,e | unreacted 1a' (%)b |
| 1 | 1.0 | 77 (78) | 90 | 25:1 | 9 | 1 |
| 2 | 0.9 | 75 (73) | 89 | 25:1 | 8 | 8 |
| 3 | 0.8 | 6 | – | 50:1 | < 1 | 94 |
| 4 | 0.7 | 4 | – | > 50:1 | < 1 | 96 |
| 5 | 0.6 | 1 | – | 50:1 | < 1 | 98 |
The catalyst 9a' was prepared by reaction of 0.5 mmol (1 equiv) of amine 6' with 10 mol% (S)-VAPOL and 30 mol% B(OPh)3 in CHCl3 for 1 h at 25 °C. The catalyst solution was added to a suspension of 150 mg of powdered 4 Å molecular sieves and x equiv of benzaldehyde to give a solution 0.5 M in amine. EDA (1.2 equiv) was added and the reaction mixture stirred for 24 h at 25 °C.
Determined from the 1H NMR spectrum of the crude reaction mixture with Ph3CH as internal standard.
Yields in parentheses are after isolation by silica gel chromatography.
Determined on purified 5a' by chiral HPLC.
Structures of 18a' and 19a' given in Table 6.
Table 6.
AZ Reaction of Pre-formed Imine 1a' with Added Bases.a
| ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | pKa | baseb | base x mol% | % yield 5a'c,d | % ee 5a'e | cis/transc | % yield 18a' + 19a'c | unreacted 1a' (%)c |
| 1 | – | none | 0 | 83 (85) | 90 | 100:1 | 10 | <1 |
| 2 | 10.75 | Et3N | 10 | 71 (77) | 86 | 25:1 | 9 | 6 |
| 3 | 20 | <1 | – | – | – | 89 | ||
| 4 | 40 | <1 | – | – | – | 92 | ||
| 5 | 10.60 | Ph2CHNH2 | 10 | 77 (80) | 89 | 27:1 | 10 | <1 |
| 6 | 20 | 10 | _ | 12:1 | 2 | 86 | ||
| 7 | 9.20 | DMAP | 10 | <1 | _ | – | – | 96 |
| 8 | 20 | <1 | – | – | – | 80 | ||
| 9 | 4.60 | C6H5NH2 | 10 | 73 (78) | 88 | 33:1 | 8 | 5 |
| 10 | 20 | 17 (20) | 86 | 23:1 | 6 | 68 | ||
Unless otherwise specified, the boroxinate catalyst 9 was prepared in CHCl3 from 10 mol% (S)-VAPOL, 30 mol% B(OPh)3 and x mol% of a base to induce boroxinate formation. After 1h at 25 °C, 100 mol% of imine 1a' and 120 mol% EDA 2 were added to the catalyst and the aziridination reaction carried out with 1.0 mmol of imine (0.5 M) for 24 h at 25 °C.
All bases were purified by distillation or sublimation.
Determined from the 1H NMR spectrum of the crude reaction mixture with Ph3CH as internal standard.
Yields in parentheses are after isolation by silica gel chromatography.
Determined on purified 5a' by chiral HPLC.
Effect of the order of addition on the multi-component aziridination
That the aziridination reaction of focus here is in fact a true multi-component reaction and in fact is a five-component catalyst assembly/catalytic asymmetric aziridination as indicated in Figure 1C is supported by the results of procedures IV and V in Table 3. The successful aziridination from the reaction where the EDA (E) is added before the aldehyde (D) reveals that imine formation can occur in the presence of EDA (procedure IV). The VAPOL ligand is not alkylated by the EDA under these conditions (Table 3, procedure IV) as might have been expected from the results in entry 6 of Table 1. This reveals that once the VAPOL boroxinate B3 catalyst 9a is generated, the VAPOL ligand is protected from alkylation.
The results from procedure V in Table 3 where the amine C is added last reveals that this is a five-component reaction where both the catalyst assembly and the aziridination reaction can be carried out simultaneously. Since the amine is added last, considerable concern arose from the results in Table 1 (entry 6) that suggest that the VAPOL ligand may be alkylated before the catalyst is generated. That is why in procedure V all five of the components were added in the order shown without solvent, and at the end, solvent was added to effect dissolution. That this provides for a successful aziridination indicates that the amine, which is added last, can quickly assemble the catalyst such that the ligand is protected from alkylation by the EDA.
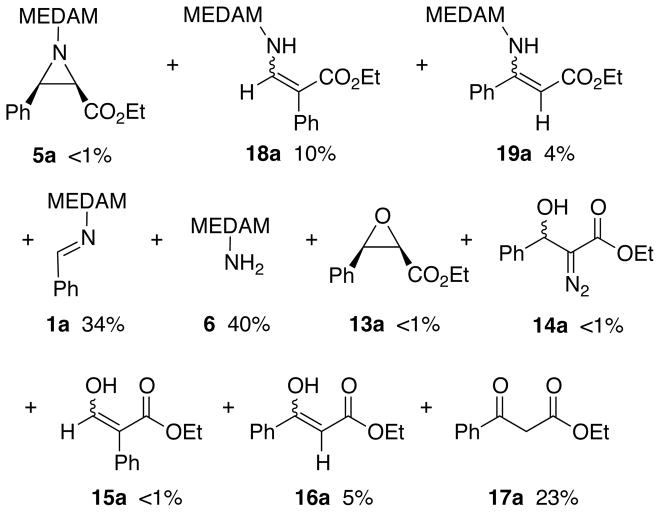
The speed at which catalyst assembly occurs is indicated by procedure VI in Table 3. When the first four components including EDA are dissolved in toluene and then 2 min later the amine is added, no aziridine formation is observed. A very complex reaction mixture is observed and the products are indicated in Figure 6 and include products from the reaction of EDA with both the aldehyde and the imine, however, no detectable amount of the aziridine 5a could be observed. The major product is the imine 1a (34%) and there is also a considerable amount of the unreacted amine 6 (40%). Since the aldehyde is partially consumed in the formation of the β-keto ester 17a (23%) and its tautomer 16a (5%), the presence of the unreacted amine 6 may lead to its selective binding to the catalyst thus preventing aziridination of the imine if indeed any boroxinate catalyst was formed at all in this reaction. As a control, the same reaction in which the amine is not added at all was also carried out (Table 2, procedure VII) and this gave a product mixture that was shown to have a very similar profile to the reaction of benzaldehyde 7a and ethyl diazoacetate 2 promoted by B(OPh)3 (Scheme 2). The combined results from procedures V and VI in Table 3 make it clear that the assembly of the catalyst by the amine is exceedingly fast. The events that occur in the two minutes prior to amine addition in procedure VI are sufficient to completely thwart catalyst assembly and aziridination.
Figure 6.
Products from the reaction with procedure VI in Table 3.
Catalyst loading, N-substituent and ligand screening
The catalytic asymmetric synthesis of aziridines from imines and diazo compounds has been honed with both benzhydryl and bis-(3,5-dimethylanisyl)methyl (MEDAM) substituents on the imine nitrogen.1–4 Thus, the multi-component aziridination was examined with both benzhydryl amine 6' and the MEDAM amine 6 and the results with benzaldehyde are shown in Table 4. In addition, the reaction of both amines were screened with both VANOL and VAPOL catalysts and the catalyst loading profile was explored as well. These reactions were screened with catalyst generated by procedure IIIA since this is experimentally the simplest to perform and it gives the same outcome as the other procedures (Tables 2 and 3). This is not always true for catalysts generated from some imines. As was found for the reactions of the corresponding imines,1b the multi-component aziridination of benzaldehyde with MEDAM amine 6 gives higher yields and asymmetric inductions than the benzhydryl amine 6'. Nonetheless, the aziridines from benzhydryl amines can in most cases be crystallized to ≥ 99% ee which is important to note upon considering that benzhydryl amine 6' is commercially available while the MEDAM amine 6 is not.1a Also as was observed with the corresponding imines, the multi-component aziridinations with VAPOL and VANOL catalysts are very similar with a slight edge in asymmetric induction to the VAPOL catalyst.1a,b The optimal catalyst loading for the reaction with the MEDAM amine is 3–5 mol%. This reaction does not go to completion in 24 h with 1 mol % of either the VANOL or VAPOL catalyst but both go to completion with a 3 mol% catalyst loading. The reaction is severly impeded by the absence of molecular sieves and gives only a 35 % yield of the aziridine 5a along with a 52% yield of the unreacted imine 1a generated from amine 6 and benzaldehyde (entry 8) and this is consistent with the previous observation that added water can greatly slow down the aziridination of imines 7a Finally, in the optimization studies it was noted that 4 Å molecular sieves was superior to 5 Å molecular sieves which gives more unknown side-products and a lower yield of the aziridine (entries 7 vs 9).
Table 4.
Catalyst Loading, N-Substituent and Ligand Screening.a
| |||||||
|---|---|---|---|---|---|---|---|
| entry | ligand | PG | catalyst x mol% | % yield 5a/5a' b | % ee 5a/5a' c | cis/transd | % yield enamined |
| 1 | (S)-VAPOL | CHPh2 | 10 | 85 | 91 | 25:1 | 9 |
| 2 | (R)-VANOL | CHPh2 | 10 | 77 | –88 | 50:1 | 15 |
| 3 | (S)-VAPOL | CHPh2 | 5 | 77 | 92 | 50:1 | 6 |
| 4 | (S)-VANOL | CHPh2 | 5 | 72 | 91 | 50:1 | 7 |
| 5 | (S)-VAPOL | MEDAM | 10 e | 90 | 98 | > 50:1 | < 1 |
| 6 | (R)-VANOL | MEDAM | 10 e | 93 | –96 | > 50:1 | < 1 |
| 7 | (S)-VAPOL | MEDAM | 5 | 98 | 98 | > 50:1 | < 1 |
| 8 | (S)-VAPOL | MEDAM | 5 | (35)f,g | – | > 50:1 | < 1 |
| 9 | (S)-VAPOL | MEDAM | 5 | 70 h | 98 | > 50:1 | 2 |
| 10 | (S)-VANOL | MEDAM | 5 | 95 | 95 | > 50:1 | < 1 |
| 11 | (S)-VAPOL | MEDAM | 3 | 92 | 98 | > 50:1 | 5 |
| 12 | (S)-VANOL | MEDAM | 3 | 94 | 97 | > 50:1 | 6 |
| 13 | (S)-VAPOL | MEDAM | 1 | (20) | – | > 50:1 | < 1 |
| 14 | (S)-VANOL | MEDAM | 1 | (31) | – | > 50:1 | < 1 |
Unless otherwise specified, all reactions were performed with procedure IIIA in Table 2 with 0.5 mmol of amine 6/6' (0.5 M) with 1.05 equiv of benzaldehyde 7a and 1.2 equiv of EDA 2 and went to 100% completion.
Isolated yield after chromatography on silica gel. Yield in parenthesis determined by 1H NMR with Ph3CH as internal standard.
Determined on purified cis-5a or cis-5a' by HPLC.
Determined by 1H NMR spectra of the crude reaction mixture.
Reaction performed with procedure IIA in Table 2.
Reaction performed without 4 Å molecular sieves.
The 1H NMR spectra of the crude reaction mixture indicates the presence of 52% unreacted imine 1a.
Reaction performed with 5 Å molecular sieves.
Sequence of the final steps in the five-component catalyst assembly/aziridination
The data in Table 3 indicates that instead there is a very rapid assembly of a boroxinate species from the VAPOL ligand and B(OPh)3 mediated by the amine 6 which gives rise to the AMINO-BOROX species 9d. At this point the question arises as to the subsequent order of events. In one extreme, a small amount of imine 1 could be formed and then immediately converted to aziridine 5 before the rest of the imine is formed (B to D in Figure 7). In the other extreme, the process could first proceed to give complete conversion of the amine and aldehyde to the imine 1 and only then does the imine 1 react with EDA 2 under the ageis of the IMINO-BOROX catalyst 9a to give the aziridine 5 (C to D in Figure 7). The last entry in Table 2 suggests that it is the latter since the presence of excess amine over aldehyde (0.6 equiv) results in the complete suppression of aziridine formation. This issue was further probed with additional experiments and the results are presented in Tables 5 and 6.
Figure 7.
Time Course of Events in the Five-component Catalyst Assembly/AZ Reaction. A Time zero. B Assembly of AMINO-BOROX 9d. C Formation of Imine 1 and IMINO-BOROX catalyst 9a. D Catalytic Asymmetric Aziridination.
The multi-component aziridination of benzaldehyde 7a with benzhydryl amine 6' was examined with different amounts of benzaldehyde ranging from 0.6 to 1.0 equiv and the results are presented in Table 5. Just as with the reaction of the MEDAM amine 6 with 0.6 equiv of benzaldehyde (Table 2, entry 6), the reaction of benzhydryl amine 6' with 0.6 equiv of benzaldehyde results in essentially no formation of aziridine 5a' (1%) even though benzaldehyde is essentially completely transformed into the imine 1a' and 98% of this imine remains unreacted after 24 h (Table 5, entry 5). The amount of aziridine 5a' formed with 0.9 equiv of benzaldehyde (entry 2) is nearly the same as with 1.0 equiv (entry 1). However, it should be noted that with 0.9 equiv, 8% of the imine 1a' remains unreacted, whereas with 1.0 equiv of benzaldehyde, the imine 1a' is nearly completely consumed (1% unreacted). This suggests there is an equilibrium between the boroxinate 9d containing a protonated amine and the boroxinate 9a containing a protonated imine (Scheme 3). Given the fact that imines are ~ 103 times less basic than amines,21 even when the amount of excess amine is equal to the amount of catalyst (0.9 equiv 7a added, entry 2), there appears to be enough of the IMINO-BOROX catalytst 9a present in solution containing the activated imine to allow the reaction to proceed in 24 h. It should be pointed out that factors other than basicity may affect the equilibrium of AMINO-BOROX and IMINO-BOROX species (see below, Table 6). Even if the equilibrium between 9d' and 9a' is facile (Figure 7), the reaction greatly slows when equal amounts of amine 6' and imine 1a' are present since the amount of unreacted imine 1a' is 8% when the reaction with 10% excess amine 6' is stopped after 24 h (entry 2). This suggests that the greater base strength of the amine 6' greatly favors the AMINO-BOROX species 9d' over that of the IMINO-BOROX 9a' when the concentrations of the amine and imine are similar. In the normal protocol for the multi-component catalytic asymmetric aziridination (Table 4), a slight excess of aldehyde (1.05 equiv) is typically used which assures complete consumption of the amine and avoidance of a slow down during the end of the reaction.
Scheme 3.
To further examine the effect of the base strength of other species present in the reaction mixture, the aziridination of the pre-formed imine 1a' was carried out in the presence of varying amounts of four different nitrogen bases and the results are presented in Table 6. In the absence of any added base, the reaction gives aziridine 5a' in 85% isolated yield and 90% ee which is nearly the same as has been previously reported for this reaction in chloroform.1a In these reactions, a boroxinate species is assembled by treating VAPOL and 3 equiv of B(OPh)3 with the base at room temperature for 1 h and then adding the imine 1a' and the EDA to consummate the reaction. These conditions directly generate the situation indicated in Figure 5C where only the imine and EDA substrates are present. The difference is that the reaction begins with an AMINO-BOROX catalyst rather than an IMINO-BOROX catalyst. The reaction in the presence of 10 mol% of benzhydryl amine 6' (Table 6, entry 5) gives nearly the same yield as the reaction without base, and this mirrors the result in entry 2 of Table 5. The only difference is that none of the imine 1a' remains at the end of the reaction with the pre-formed imine. However, the reaction with 20 mol% of benzhydryl amine 6' slows the reaction dramatically and 86% of the imine 1a' remains after 24 h. Similar results are seen with 10 mol% Et3N and 10 mol% benzhydryl amine 6' (entries 2 vs 5), however, with 20 mol% Et3N the reaction is completely stopped and no detectable amount of the aziridine is observed (entries 2 & 3).
Aniline is a weaker base than aliphatic amines and this can be correlated with its decreased ability to slow the reaction since even with 20 mol% aniline there is 32% conversion of the imine giving a 20% isolated yield of aziridine 5a' (entry 10). These results further suggest that an equilibrium is present between an AMINO-BOROX and an IMINO-BOROX species (Scheme 3). The fact that there is nearly complete reaction when 10 mol% Et3N or 10 mol% Ph2CHNH2 are added, suggests that even when there is a 1:1 mixture of the AMINO-BOROX and the IMINO-BOROX species there is still enough of the IMINO-BOROX species present such that the reaction can still go forward. To this point, the reader’s attention should be brought to the fact that although these reactions were performed with 10 mol% catalyst, the aziridination reaction with benzhydryl imines can be effected under these conditions with as little as 0.25 mol% catalyst.1a
The ability of the amine to inhibit the reaction does correlate to some degree with the basicity of the amine, but there are obviously other factors that must contribute to the magnitude of the equilibrium constant indicated in Scheme 3. This is most clear in the reaction with added DMAP. Although less basic than aliphatic amines, DMAP completely stops the reaction upon addition of just 10 mol% (Table 6, entry 7). In this case, any equilibrium between the AMINO-BOROX and IMINO-BOROX species may be determined by more than just the relative basicity of the amine and the imine. This equilibrium may be shifted to the AMINO-BOROX further than might be predicted from the basicity alone. The crystal structure of the AMINO-BOROX species containing DMAP reveals the presence of non-covalent interactions in addition to the H-bond between the protonated base and the boroxinate anion.7b Specifically, there is a π-π stacking interaction between the pyridinium cation and one of the phenanthrene rings of the VAPOL ligand and there is a CH-π interaction between one of the ortho-hydrogen of the pyridinium cation and one of the phenoxy groups of the boroxinate core.7b These results are consistent with the view that the catalytic cycle for the multi-component catalyst assembly/aziridination involves the complete (or nearly complete) formation of the imine before the aziridination reaction can commence (Figure 5B to 5C).
Conclusions
The practicing chemist now has at one’s avail a multi-component asymmetric catalytic method for the preparation of aziridines directly from aldehydes, an amine and a diazo compound.5 The chiral catalyst for this reaction has a boroxinate core comprised of a chiral polyborate that is assembled in-situ from a vaulted biaryl ligand (VANOL or VAPOL) and three equivalents of B(OPh)3 by the amine substrate. The present work shows that this is actually a five-component process involving both catalyst assembly and a catalytic asymmetric aziridination reaction where the catalyst is assembled by one of the substrates before it is consumed. A solution of these five components has the potential to create a chaotic array of products and yet there is order which leads to the formation of aziridines in very high yields, diastereoselectivities and enantioselectivities. This order is imposed by the amine in causing an exceedingly fast assembly of the boroxinate catalyst from the ligand and B(OPh)3. Once the catalyst is assembled, it is protected from alkylation by the diazo compound and since the B(OPh)3 is consumed, the epoxidation reaction of the diazo compound and the aldehyde is thwarted. This then permits the formation of an imine from the aldehyde and amine and the resulting imine binds in its protonated form to the boroxinate catalyst. This is followed by binding of the diazo compound to the chiral boroxinate catalyst in such proximity to the bound iminium that an aziridine is formed with high diastereo- and enantioselection.3b
3. Experimental Section
General procedure for multi-component aziridination with benzaldehyde
(2R,3R)-ethyl-1-(bis(4-methoxy-3,5-dimethylphenyl)methyl)-3-phenylaziridine-2-carboxylate 5a (Table 4, entry 7): To a 10 mL flame-dried single-necked round bottom flask equipped with a stir bar and filled with argon was added (S)-VAPOL (14 mg, 0.025 mmol), B(OPh)3 (22 mg, 0.075 mmol) and amine 6 (149.7 mg, 0.5000 mmol). Dry toluene (1 mL) was added under an argon atmosphere to dissolve the reagents. The flask was fitted with a rubber septum and a nitrogen balloon. The reaction mixture was stirred at room temperature for 1 h. Thereafter, 4Å Molecular Sieves (150 mg, freshly flame-dried) was added followed by the addition of the aldehyde 7a (52.0 μL, 0.525 mmoL, 1.05 equiv). To this solution was rapidly added ethyl diazoacetate (EDA) 2 (62 μL, 0.60 mmoL, 1.2 equiv). The resulting mixture was stirred for 24 h at room temperature. The reaction was diluted by addition of hexane (6 mL). The reaction mixture was then filtered through a Celite pad to a 100 mL round bottom flask. The reaction flask was rinsed with EtOAc (3 mL × 3) and the rinse was filtered through the same Celite pad. The resulting solution was then concentrated in vacuo followed by exposure to high vacuum (0.05 mm Hg) for 1 h to afford the crude aziridine as an off-white solid.
The cis/trans ratio was determined by comparing the 1H NMR integration of the ring methine protons for each aziridine in the crude reaction mixture. The cis (J = 7–8 Hz) and the trans (J = 2–3 Hz) coupling constants were used to differentiate the two isomers. The yields of the acyclic enamine side products 18a and 19a were determined by 1H NMR analysis of the crude reaction mixture by integration of the N-H proton relative to the that of the cis-aziridine methine protons with the aid of the isolated yield of the cis-aziridine. Purification of the crude aziridine by silica gel chromatography (30 mm × 300 mm column, 9:1 hexanes/EtOAc as eluent, gravity column) afforded pure cis-aziridine 5a as a white solid (mp 107–108 ºC on 99.8% ee material) in 98% isolated yield (230 mg, 0.490 mmol); cis/trans: >50:1. Enamine side products: <1% yield of 18a and <1% yield of 19a. The optical purity of 5a was determined to be 98% ee by HPLC analysis (CHIRALCEL OD-H column, 99:1 hexane/2-propanol at 226nm, flow-rate: 0.7 mL/min): retention times; Rt = 9.26 min (major enantiomer, 5a) and Rt = 12.52 min (minor enantiomer, ent-5a). Spectral data for 5a: Rf = 0.42 (1:9 EtOAc/hexane); 1H NMR (CDCl3, 500 MHz) δ 0.98 (t, 3H, J = 7.1 Hz), 2.18 (s, 6H), 2.24 (s, 6H), 2.55 (d, 1H, J = 6.8 Hz), 3.10 (d, 1H, J = 6.6 Hz), 3.62 (s, 3H), 3.66 (s, 1H), 3.68 (s, 3H) 3.87–3.97 (m, 2H), 7.09 (s, 2H), 7.18 (s, 2H), 7.21–7.24 (m, 3H), 7.36 (d, 2H, J = 7.3 Hz); 13C (CDCl3, 125 MHz) δ 14.0, 16.16, 16.22, 46.3, 48.2, 59.5, 59.6, 60.5, 77.0, 127.2, 127.4, 127.7, 127.8, 127.9, 130.59, 130.60, 135.3, 137.8, 138.0, 156.0, 156.1, 168.0; IR (thin film) 2961 vs, 1750 vs, 1414 vs, 1202 vs cm−1; Mass spectrum: m/z (% rel intensity) 473 M+ (0.27), 284(78), 283 (100), 268 (34), 253 (20), 237 (11), 210(10), 117 (18), 89 (11); Anal calcd for C30H35NO4: C, 76.08; H, 7.45; N, 2.96. Found: C, 76.31; H, 7.28; N, 2.82; [α]23D +41.3 (c 1.0, EtOAc) on 99% ee material (HPLC). These spectral data match those previously reported for this compound.1c
(2R,3R)-ethyl 1-benzhydryl-3-phenylaziridine-2-carboxylate 5a' (Table 4, entry 3):Aldehyde 7a (52.0 μL, 0.525 mmol, 1.05 equiv) was reacted according to the general Procedure described above with (S)-VAPOL as ligand. Purification of the crude aziridine by silica gel chromatography (30 mm × 300 mm column, 19:1 hexanes/EtOAc as eluent, gravity column) afforded pure cis-aziridine 5a' as a white solid (mp. 127.5–128.5 °C) in 77 % isolated yield (138 mg, 0.390 mmol); cis/trans: 50:1. Enamine side products: 6 % yield of enamines. The optical purity of 5a' was determined to be 92% ee by HPLC analysis (CHIRALCEL OD-H column, 90:10 hexane/2-propanol at 226 nm, flow-rate: 0.7 mL/min): retention times; Rt = 9.01 min (major enantiomer, 5a') and Rt = 4.67 min (minor enantiomer, ent-5a'). Spectral data for 5a': Rf = 0.3 (1:9 EtOAc/hexanes); 1H NMR (CDCl3, 500 MHz) δ 0.95 (t, 3H, J = 7.3 Hz), 2.64 (d, 1H, J = 6.8 Hz), 3.19 (d, 1H, J = 6.8 Hz), 3.91 (q, 2H, J = 7.1 Hz), 3.93 (s, 1H), 7.16–7.38 (m, 11H), 7.47 (d, 2H, J = 7.1 Hz), 7.58 (d, 2H, J = 7.6 Hz); 13C NMR (CDCl3, 125 MHz) δ 13.9, 46.4, 48.0, 60.6, 77.7, 127.2, 127.3, 127.4, 127.5, 127.8, 127.8, 128.5, 135.0, 142.4, 142.5, 167.8 (one sp2 carbon not located); [α]20D = + 33.4 (c 1.0, CH2Cl2) on 91% ee material (HPLC). These spectral data match those previously reported for this compound.1a
Supplementary Material
Figure 8.
Chemoselectivity in the 5-component catalyst assembly/catalytic asymmetric aziridination
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (GM 094478).
Footnotes
Supporting Information: NMR analysis of boroxinate catalyst formation and of reactions of 7a with 2, order of addition and stoichiometry studies and DFT calculations and pdf files for 9a and 9b. This material is available free of charge via the internet at http://pubs.acs.org/.
References
- 1.a) Zhang Y, Desai A, Lu Z, Hu G, Ding Z, Wulff WD. Chem –Eur J. 2008;14:3785–3803. doi: 10.1002/chem.200701558. [DOI] [PubMed] [Google Scholar]; b) Mukherjee M, Gupta AK, Lu Z, Zhang Y, Wulff WD. J Org Chem. 2010;75:5643–5660. doi: 10.1021/jo101160c. [DOI] [PubMed] [Google Scholar]
- 2.Huang L, Zhang Y, Staples RJ, Huang RH, Wulff WD. Chem –Eur J. 2012;18:5302–5313. doi: 10.1002/chem.201102520. [DOI] [PubMed] [Google Scholar]
- 3.a) Desai AA, Wulff WD. J Am Chem Soc. 2010;132:13100–13103. doi: 10.1021/ja1038648. [DOI] [PubMed] [Google Scholar]; b) Vetticatt MJ, Desai AA, Wulff WD. J Am Chem Soc. 2010;132:13104–13107. doi: 10.1021/ja103863j. [DOI] [PubMed] [Google Scholar]
- 4.For a review, see: Zhang Y, Lu Z, Wulff WD. SynLett. 2009:2715–2739.
- 5.Gupta AK, Mukherjee M, Wulff WD. Org Lett. 2011;13:5866–5869. doi: 10.1021/ol202472z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.This is a five-component process involving five different components, but a seven-component process in terms of the total number of components (3 molecules of B(OPh)3).
- 7.a) Hu G, Gupta AK, Huang RH, Mukherjee M, Wulff WD. J Am Chem Soc. 2010;132:14669–14675. doi: 10.1021/ja1070224. [DOI] [PubMed] [Google Scholar]; b) Hu G, Huang L, Huang RH, Wulff W. J Am Chem Soc. 2009;131:15615–15617. doi: 10.1021/ja904589k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman CA, Antilla JC, Chen P, Wulff WD. J Am Chem Soc. 2007;129:7216–7217. doi: 10.1021/ja069019d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.We have observed before that DMAP can cause the formation of a boroxinate species from VAPOL and B(OPh)3 (reference 7b).
- 10.The amount of B1 and B2 varies with the purity of commercial B(OPh)3 which is partially hydrolyzed. Purified B(OPh)3 does not react with VAPOL to give any detectable amounts of B1 or B2.7a Commercial B(OPh)3 was used in Table 1.
- 11.This product is sometimes observed at the end of an aziridination reaction (reference 1a).
- 12.Frisch MJ, GWT, Schlegel HB, Scuseria GE, Robb MA, JRC, Montgomery JA, Jr, Vreven T, Kudin KN, JCB, Millam JM, Iyengar SS, Tomasi J, Barone V, BM, Cossi M, Scalmani G, Rega N, Petersson GA, HN, Hada M, Ehara M, Toyota K, Fukuda R, JH, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, MK, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, CA, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, AJA, Cammi R, Pomelli C, Ochterski JW, Ayala PY, KM, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, SD, Daniels AD, Strain MC, Farkas O, DKM, Rabuck AD, Raghavachari K, Foresman JB, JVO, Cui Q, Baboul AG, Clifford S, Cioslowski J, BBS, Liu G, Liashenko A, Piskorz P, Komaromi I, RLM, Fox DJ, Keith T, Al-Laham MA, Peng CY, AN, Challacombe M, Gill PMW, Johnson B, WC, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision D.01. Gaussian, Inc; Wallingford CT: 2004. [Google Scholar]
- 13.For reviews, see: Nishio M. Tetrahedron. 2005;61:6923–6950.Nishio MHM, Umezawa Y. The CH/π, Interaction: Evidence, Nature, and Consequences. Wiley-VCH; 1998.
- 14.Plevin MJ, Bryce DL, Boisbourier J. J Nat Chem. 2010;2:466. doi: 10.1038/nchem.650. [DOI] [PubMed] [Google Scholar]
- 15.BHandHLYP is considered to be a better method for the case of hydrogen bonding interactions, see: Zhao Y, Truhlar DG. J Chem Theory Comput. 2005;1:415. doi: 10.1021/ct049851d.Yu W, Liang L, Lin Z, Ling S, Haranczyk M, Gutowski M. J Comput Chem. 2009;30:589. doi: 10.1002/jcc.21091.
- 16.(a) Li W, Wang J, Hu X, Shen K, Wang W, Chu Y, Lin L, Liu X, Feng X. J Am Chem Soc. 2010;132:8532–8533. doi: 10.1021/ja102832f. [DOI] [PubMed] [Google Scholar]; (b) Hashimoto T, Miyamoto H, Naganawa Y, Maruoka K. J Am Chem Soc. 2009;131:11280–11281. doi: 10.1021/ja903500w. [DOI] [PubMed] [Google Scholar]; (c) Liu WJ, Lv BD, Gong LZ. Angew Chem Int Ed. 2009;48:6503–6506. doi: 10.1002/anie.200903061. [DOI] [PubMed] [Google Scholar]; (d) Trost BM, Malhotra S, Fried BA. J Am Chem Soc. 2009;131:1674–1675. doi: 10.1021/ja809181m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Hashimoto T, Naganawa Y, Maruoka K. J Am Chem Soc. 2008;130:2434–2435. doi: 10.1021/ja710532j. [DOI] [PubMed] [Google Scholar]; (f) Hasegawa K, Arai S, Nishida A. Tetrahedron. 2006;62:1390–1401. [Google Scholar]; (g) Dudley ME, Morshed MM, Brennan CL, Islam MS, Ahmad MS, Atuu MR, Branstetter B, Hossain MM. J Org Chem. 2004;69:7599–7608. doi: 10.1021/jo0489418. [DOI] [PubMed] [Google Scholar]; (h) Yao W, Wang J. Org Lett. 2003;5:1527–1530. doi: 10.1021/ol0343257. [DOI] [PubMed] [Google Scholar]; (i) Jiang N, Wang J. Tetrahedron Lett. 2002;43:1285–1287. [Google Scholar]; (j) Mahmood SJ, Hossain MM. J Org Chem. 1998;63:3333–3336. [Google Scholar]; (k) Holmquist CR, Roskamp EJ. J Org Chem. 1989;54:3258–3260. [Google Scholar]
- 17.(a) Ramon DJ, Yus M. Angew Chem Int Ed. 2005;44:1602–1634. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]; (b) Zhu J, Bienayme H, editors. Multicomponent Reactions. Wiley-VCH; Weinheim: 2005. [Google Scholar]; (c) de Graaff C, Ruijter E, Orru RVA. Chem Soc Rev. 2012;41:3969–4009. doi: 10.1039/c2cs15361k. [DOI] [PubMed] [Google Scholar]; (d) Dömling A, Wang W, Wang K. Chem Rev. 2012;112:3083. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.For multi-component aziridinations with non-chiral catalysts, see: Nagayama S, Kobayashi S. Chem Lett. 1998:685–686.Kubo T, Sakaguchi S, Ishii Y. Chem Commun. 2000:625–626.Yadav JS, Reddy BVS, Rao MS, Reddy PN. Tetrahedron Lett. 2003;44:5275–5278.Yadav JS, Reddy BVS, Reddy PN, Rao MS. Synthesis. 2003:1387–1390.Ishii Y, Sakaguchi S. Bull Chem Soc Jpn. 2004;77:909–920.
- 19.For a sequential component aziridination with a chiral catalyst, see: Akiyama T, Suzuki T, Mori K. Org Lett. 2009;11:2445–2447. doi: 10.1021/ol900674t.
- 20.For a stoichiometric asymmetric multi-component aziridination, see: Minakata S, Ando T, Nishimura M, Ryu I, Komatsu M. Angew Chem Int Ed. 1998;37:3392–3394. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3392::AID-ANIE3392>3.0.CO;2-G.Nishimura M, Minakata S, Takahashi T, Oderaotoshi Y, Komatsu M. J Org Chem. 2002;67:2101–2110. doi: 10.1021/jo016146d.
- 21.Favrot J, Vocelle D, Sandorfy C. Photochem and Photobiol. 1979;30:417–421. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.