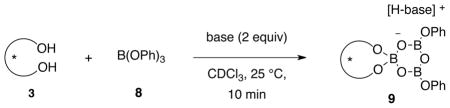
Table 1.
Base Induced Boroxinate Formation from (S)-VAPOL and B(OPh)3.a
| |||||||
|---|---|---|---|---|---|---|---|
| entry | base | (S)-VAPOL 3 (% unreacted)b | % yield 10 (B1)c | % yield 11 (B2)d | % yield 9 (B3) | 9 | 9 (B3) δ (ppm) (1H; 11B)e |
| 1 | Et3N | 8 | <1 | <1 | 63 | 9b | 10.25, 5.37 |
| 2 | i-Pr2NH | <1 | <1 | <1 | 74 | 9c | 10.31, 5.18 |
| 3 | Ar2CHNH2 f | 3 | <1 | <1 | 50 | 9d | 10.37, 5.48 |
| 4 | Ar2CHN=CHPh f,g | 11 | <1 | <1 | 76 | 9a | 10.31, 5.51 |
| 5 | C6H5NH2 | 24 | <1 | <1 | 61 | 9e | 10.29, 5.56 |
| 6 | N2CHCO2Et h | 15 | <1 | <1 | <1 | 9f | – – – – |
| 7 | MeCONMe2 | 32 | <1 | 24 | 18 | 9g | 10.15, 5.78 |
| 8 | t-BuCHO | 37 | <1 | 33 | <1 | 9h | – – – – |
| 9 | C6H5CHO | 52 | <1 | 27 | <1 | 9i | – – – – |
| 10 | no base | 57 | 5 | 18 | <1 | 9j | – – – – |
0.20 mmol of base where added to a solution of 0.10 mmol of (S)-VAPOL and 0.30 mmol of commercial B(OPh)3 in 1.0 mL of CDCl3 at 25 °C and allowed to stir 10 min before spectra were taken. All yields are 1H NMR yields with Ph3CH as internal standard.
Bay region doublet at δ = 9.77 ppm.
Bay region doublet at δ = 9.55 ppm.
Bay region doublet at δ = 9.22 ppm.
Chemical shifts for bay region doublet in 1H NMR and four-coordinate boron in 11B NMR.
Reference 6a.
This reaction produces an 80% yield of the mono-alkylation product 12 (Scheme 2).
