Table 3.
Effects of Order of Addition on the Multi-Component Aziridination.a
| procedure | reaction protocol | % yield 5a b | % ee 5a c | |
|---|---|---|---|---|
| IVd | A + B + C + E |
|
94 | 98 |
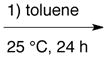
| Ve |
A + B + D* + E + C * plus 4 Å MS |
|
85 f | 98 |
| VIg |
A + B + D* + E * plus 4 Å MS |
|
<1 | – |
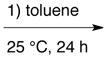
| VIIh |
A + B + D* + E *plus 4 Å MS |
|
– | – |
All reactions were performed as described in Table 2.
Isolated yield after chromatography on silica gel. Analysis of the crude reaction mixture indicates =50:1 cis:trans selectivity for 5a.
Determined on purified cis-5a by HPLC.
The diazo compound E is added to a mixture of A, B and C and then toluene is added and the mixture stirred at 25 °C for 1 h after which 4 Å powdered molecular sieves and D are added and the mixture stirred for 24 h at 25 °C.
A mixture of A, B, 4 Å MS, D, E and C, added in that order, was dissolved in toluene and the resulting solution allowed to stir for 24 h at 25 °C.
A 3% yield of enamines 18a and 19a was observed.
A mixture of A, B, 4 Å MS, D and E, added in that order, was dissolved in toluene and the resulting solution allowed to stir for 2 min at 25 °C before C was added and the resulting solution stirred for 24 h at 25 °C. The products of this reaction are shown in Figure 6.
Same as procedure VI except that C was not added. The products from this reaction are 13a (5%), 14a (<1%), 15a (5%), 16a (11%), 17a (38%) and 12 (37%).
