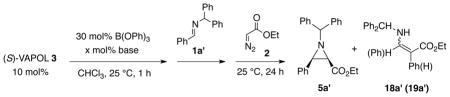
Table 6.
AZ Reaction of Pre-formed Imine 1a' with Added Bases.a
| ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | pKa | baseb | base x mol% | % yield 5a'c,d | % ee 5a'e | cis/transc | % yield 18a' + 19a'c | unreacted 1a' (%)c |
| 1 | – | none | 0 | 83 (85) | 90 | 100:1 | 10 | <1 |
| 2 | 10.75 | Et3N | 10 | 71 (77) | 86 | 25:1 | 9 | 6 |
| 3 | 20 | <1 | – | – | – | 89 | ||
| 4 | 40 | <1 | – | – | – | 92 | ||
| 5 | 10.60 | Ph2CHNH2 | 10 | 77 (80) | 89 | 27:1 | 10 | <1 |
| 6 | 20 | 10 | _ | 12:1 | 2 | 86 | ||
| 7 | 9.20 | DMAP | 10 | <1 | _ | – | – | 96 |
| 8 | 20 | <1 | – | – | – | 80 | ||
| 9 | 4.60 | C6H5NH2 | 10 | 73 (78) | 88 | 33:1 | 8 | 5 |
| 10 | 20 | 17 (20) | 86 | 23:1 | 6 | 68 | ||
Unless otherwise specified, the boroxinate catalyst 9 was prepared in CHCl3 from 10 mol% (S)-VAPOL, 30 mol% B(OPh)3 and x mol% of a base to induce boroxinate formation. After 1h at 25 °C, 100 mol% of imine 1a' and 120 mol% EDA 2 were added to the catalyst and the aziridination reaction carried out with 1.0 mmol of imine (0.5 M) for 24 h at 25 °C.
All bases were purified by distillation or sublimation.
Determined from the 1H NMR spectrum of the crude reaction mixture with Ph3CH as internal standard.
Yields in parentheses are after isolation by silica gel chromatography.
Determined on purified 5a' by chiral HPLC.
