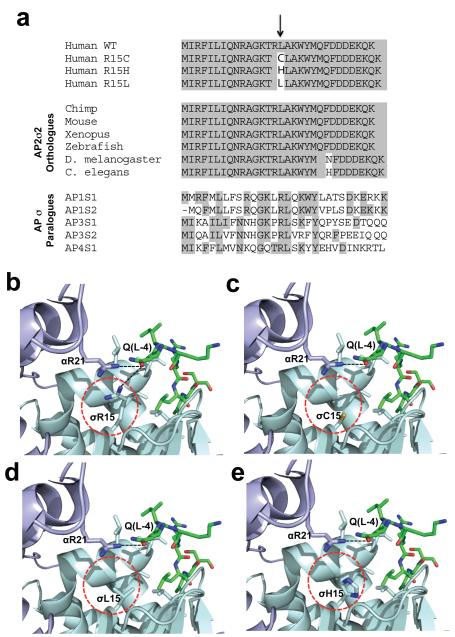
Figure 1. Evolutionary conservation of AP2σ2 Arg15, and structural analysis of mutants.
a, Multiple protein sequence alignment of AP2σ2 revealed evolutionary conservation of Arg15 (R15) residues in orthologues and human paralogues. Conserved residues are shaded grey. The Arg15 residue is conserved in all AP2σ2 subunits from human to C. elegans and in all human AP sigma subunit paralogues. b, Location of Arg15 residue in crystal structure of AP2 heterotetramer bound to an acidic dileucine motif4. Polar contacts (black dotted lines) of the Arg15 residue of AP2σ2 (pale-blue) and Arg21 (R21) residue of AP2α1 (mauve) with the glutamine (Q(L-4)) residue of the acidic dileucine peptide (green) are indicated. The AP2σ2 mutants are circled red. The mutants c, Cys15 (C15), d, Leu15 (L15)and e, His15 (H15) are predicted to result in the loss or weakening of this key polar contact.