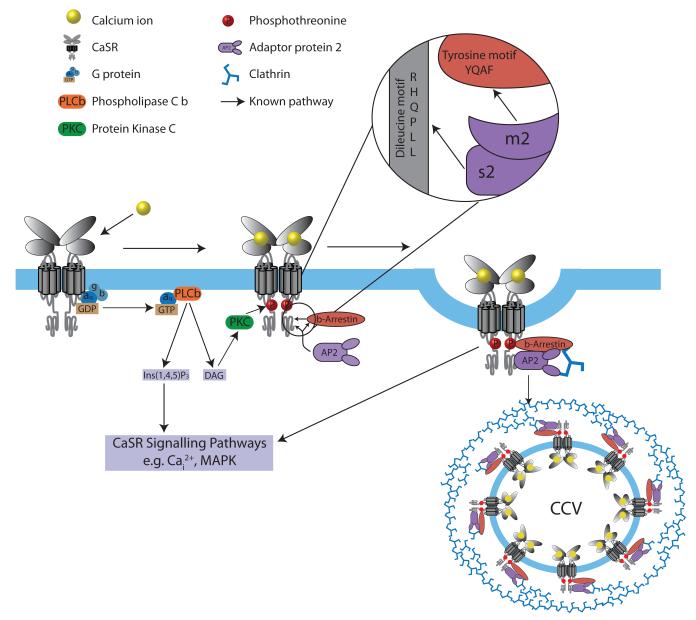
Figure 2. Schematic model for CaSR signaling and its endocytosis mediated by β-arrestin and AP2.
Ligand binding of calcium ions (yellow) by the GPCR CaSR (grey) results in G-protein-dependent stimulation, through Gq/11, of phospholipase C (PLCβ)(orange) activity, causing an accumulation of inositol 1,4,5-trisphosphate (IP3) and rapid release of calcium ions from intracellular stores (Cai2+), as well as an increase in diacylglycerol (DAG) and stimulation of Protein Kinase C (PKC) which phosphorylates CaSR threonine residues (red P) that promotes binding by β-arrestin (brown) and initiates CaSR internalization10,15,17,28. AP2 (purple) plays a pivotal role in initiating GPCR internalisation by binding the β-arrestin tyrosine (YxxΦ) motif, via its μ2 subunit; indeed, mutations of the AP2 μ2 subunit disrupt binding to the β-arrestin tyrosine motif and severely impair clathrin-mediated endocytosis29. AP2, which is a central component of clathrin-coated vesicles (CCVs), links clathrin to the vesicle membrane and binds to the tyrosine-based and dileucine-based motifs of membrane-associated cargo proteins1,3. Internalised GPCR-β-arrestin complexes may signal by G protein-independent pathways e.g. mitogen-activated protein kinase (MAPK) signaling10,17. Calcium-stimulation decreases CaSR cell-surface expression20, and AP2σ2 may facilitate this CaSR internalisation by interaction with a C-terminal dileucine motif (Arg-His-Gln-Pro-Leu-Leu)(RHQPLL)) of the CaSR; indeed studies of the related GPCR, γ-aminobutyric acid type B (GABAB) receptor have shown that removal of this dileucine motif impairs internalisation of this GPCR30.