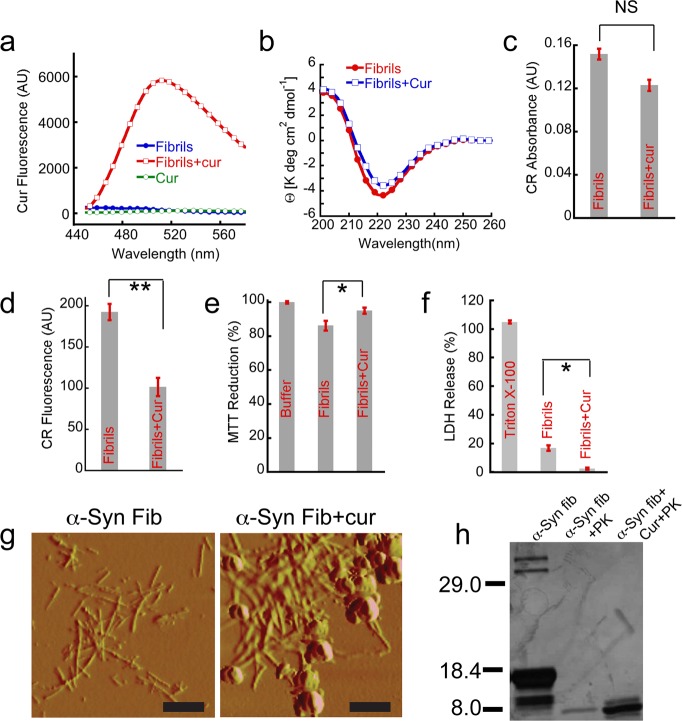
Figure 3.
Curcumin binds to α-Syn fibrils and reduces their toxicity. 100 μM preformed α-Syn fibrils were incubated with and without 100 μM curcumin for 20 h, and these samples were used for all the assays. (a) Significant increase in curcumin fluorescence at ∼500 nm was observed after binding to fibrils when excited at 426 nm. (b) CD spectra of preformed α-Syn fibrils without (red) and with curcumin (blue). Both the spectra showed mostly β-sheet conformation without any significant change. (c) CR absorbance at 540 nm after binding to α-Syn fibrils in the presence and absence of curcumin. (d) CR fluorescence at 595 nm showing decreased in CR binding when preformed α-Syn fibrils were incubated in the presence of curcumin. (e) MTT reduction assay using SH-SY5Y cell line by preformed α-Syn fibrils in the presence and absence of curcumin. (f) LDH release assay using SH-SY5Y cells showing less LDH release by fibrils incubated in the presence of curcumin. 0.5% Triton X-100 used as a positive control and showed 100% cell death. (g) AFM morphology of α-Syn fibrils in the presence and absence of curcumin. Left panel shows the distinct fibrillar morphology of α-Syn amyloids, whereas the right panel shows clustered and clumped fibrillar aggregates along with some amorphous species when α-Syn fibrils were incubated with curcumin. Scale bars are 500 nm. (h) Altered proteinase K digestion profile evident from SDS-PAGE analysis showing α-Syn fibrils in the presence of curcumin is more resistant to proteolytic degradation. Statistical significance *P < 0.05; **P < 0.01; NS P > 0.05.