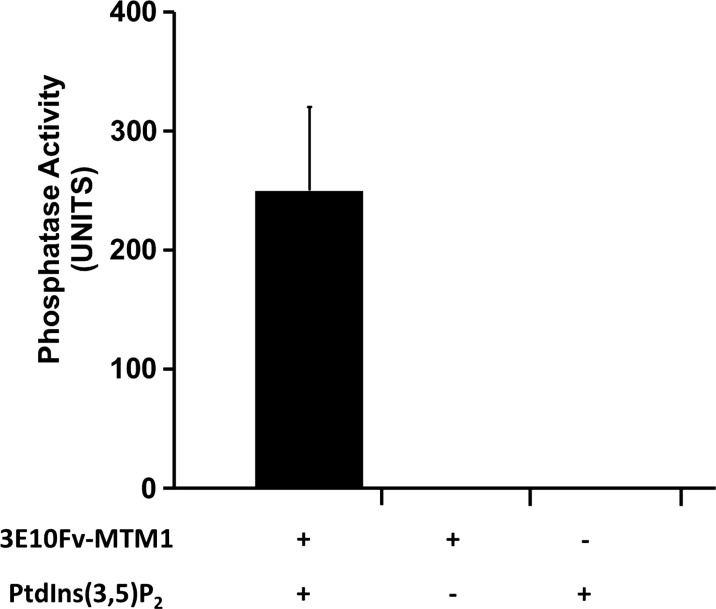
Figure 5.
3E10Fv-MTM1 possesses intact phosphatase activity. A malachite green phosphatase activity assay confirms the phosphatase activity of 100 nM 3E10Fv-MTM1expressed in, and purified from E. coli toward 50 µm PtdIns (3,5)P2 over a period of 20 min at 37°C as measured in n = 6 reactions over two experiments. An average derived from six reactions over two experiments measured in triplicate demonstrated a mean Pi generation of 249.83 ± 70.07 pmoles per 20 µl reaction over the course of 20 min, whereas reactions deficient in enzyme and/or substrate showed no detectable Pi release. Compared with previously published data on MTM1-alone phosphatase activity (52,53), the 3E10Fv-MTM1 protein showed comparable calculated activity, especially, when considering the proportional molecular weight of myotubularin as a percentage (∼70%) of the entire 3E10Fv-MTM1 fusion protein.