Figure 1.
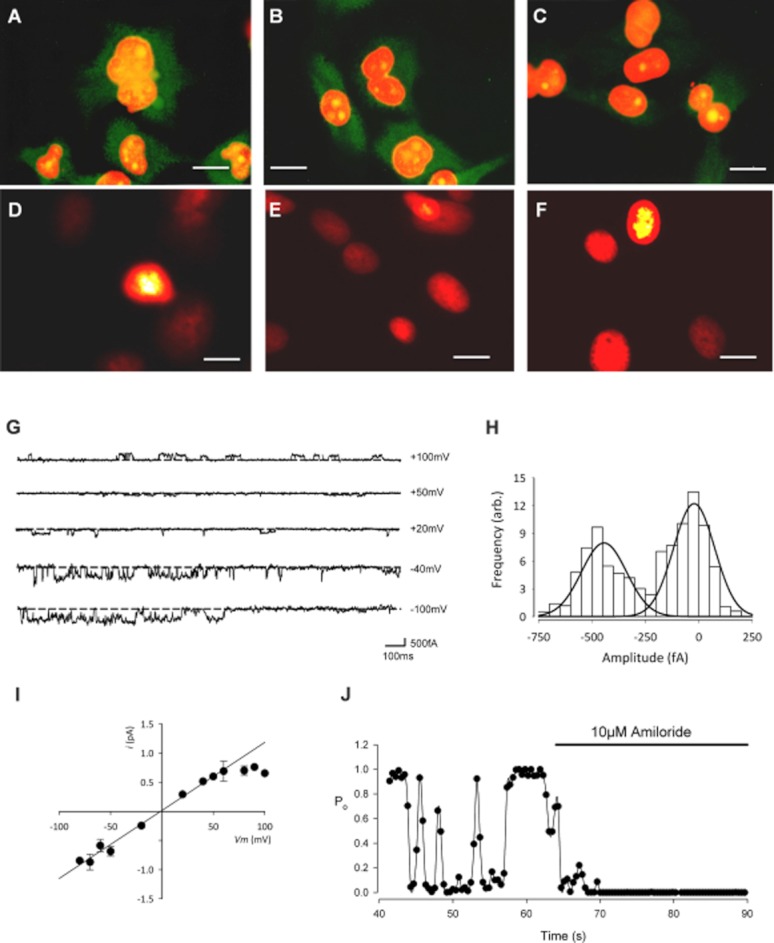
Immunohistochemistry and patch-clamp recording reveal the presence of ENaC protein expression and a benzamil and amiloride sensitive cation conductance. Immunofluorescence staining of αENaC (A), βENaC (B) and γENaC (C) in primary cultures of canine articular chondrocytes (first passage cells, 100× objective). Positive immunoreactivity for all subunits was observed although strongest for α- and β-ENaC. The concentration of the primary antibodies was 1 μg·mL−1 (dilution 1:200). The secondary antibody was a goat polyclonal to rabbit IgG (Fc-specific, affinity purified, pre-adsorbed) conjugated to DyLight® 488 (Abcam ab98462). After extensive washes in PBS-T, the nuclei were counterstained with propidium iodide (red fluorescence). Green staining shows the ENaC protein, orange staining is the nuclei and yellow staining is the overlap of the two. (D–F) The negative control was treated identically, but with the omission of primary IgG and shows the red fluorescent nuclear staining with very little background green fluorescence (scale bars for A–F: 10 μm). (G–J) Inside-out patch clamp recordings from canine chondrocytes using inside-out solutions: Tables 1 and 2. (G) Traces of inside-out low conductance single channel activity at the given membrane potentials. The scale bar horizontal line is 100 ms; vertical line is 500 fA. (H) All-points amplitude histogram for the low conductance channel at −40 mV. (I) Single channel current–voltage curve for the low conductance channel. Vrev was −1 ± 5 mV (n = 5), slope conductance 9 ± 1 pS (n = 5). (J) Open probability (Po) versus time, calculated over successive 0.4 s windows before and during the addition of the ENaC channel inhibitor, amiloride (10 μM). Low conductance single channel Po was reduced by 96 ± 2% (n = 5).
