Figure 3.
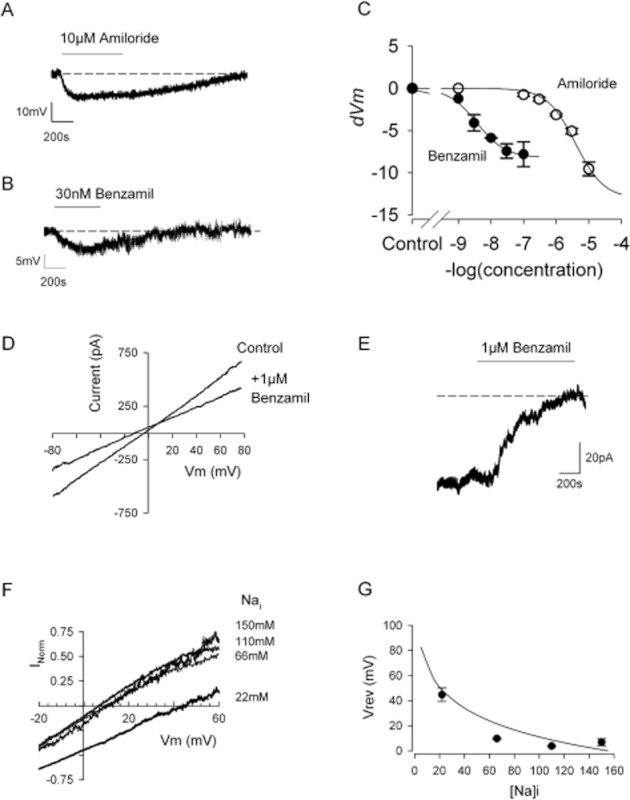
Whole-cell patch clamp shows the presence of a sodium conductance characteristic of ENaC. (A and B) Whole-cell current clamp recordings of a canine chondrocyte in ‘physiological saline solutions’ (Tables 1 and 2, including 145 mM external NaCl). Application of either amiloride (A) or benzamil (B) reversibly hyperpolarize the membrane. Mean values given in the text. (C) Comparison of the effect of amiloride and benzamil on membrane potential in a number of experiments such as those shown in panels A and B. Data are fitted with Equation 3, where R is the change in membrane potential (dVm). Hill slope (h) was constrained to unity, m for amiloride was −13 ± 1.2 mV and pD2 = 5.5 ± 0.1, for benzamil m was −8.2 ± 0.3 mV and pD2 = 8.4 ± 0.07 (benzamil from eight cells, amiloride each point, three to five cells). (D) Representative whole-cell voltage ramps in ‘ENaC permeability’ solutions (Tables 1 and 2). The current traces shown illustrate a recording in control and then 1 μM benzamil solution. The resulting difference currents for each combination of solutions is shown in panel F. (E) Representative continuous whole-cell voltage clamp recordings as a chondrocyte is superfused with 1 μM benzamil. The small constitutive inward current is blocked resulting in an increase in outward current corresponding to a mean total whole-cell conductance of 1.5 ± 0.4 nS (n = 9). (F) Mean benzamil difference currents with 22 (49), 66 (13), 110 (6) and 150 (7) mM intracellular sodium (calculated Na equilibrium potential in mV) are shown (n = 14). (G) Mean Vrev plotted against intracellular sodium concentration. The smooth line represents a fit to Equation 2 with P (PNa/PK) of 24.
