Abstract
We report in this study an intrinsic link between pyrimidine metabolism and liver lipid accumulation utilizing a uridine phosphorylase 1 transgenic mouse model UPase1-TG. Hepatic microvesicular steatosis is induced by disruption of uridine homeostasis through transgenic overexpression of UPase1, an enzyme of the pyrimidine catabolism and salvage pathway. Microvesicular steatosis is also induced by the inhibition of dihydroorotate dehydrogenase (DHODH), an enzyme of the de novo pyrimidine biosynthesis pathway. Interestingly, uridine supplementation completely suppresses microvesicular steatosis in both scenarios. The effective concentration (EC50) for uridine to suppress microvesicular steatosis is approximately 20 µM in primary hepatocytes of UPase1-TG mice. We find that uridine does not have any effect on in vitro DHODH enzymatic activity. On the other hand, uridine supplementation alters the liver NAD+/NADH and NADP+/NADPH ratios and the acetylation profile of metabolic, oxidation-reduction, and antioxidation enzymes. Protein acetylation is emerging as a key regulatory mechanism for cellular metabolism. Therefore, we propose that uridine suppresses fatty liver by modulating the liver protein acetylation profile. Our findings reveal a novel link between uridine homeostasis, pyrimidine metabolism, and liver lipid metabolism.
Keywords: CARS microscopy, fatty liver, hepatic microvesicular steatosis, liver lipid metabolism, protein acetylation, uridine phosphorylase
Nucleotides are building blocks of nucleic acids RNA and DNA (1). In addition to serving as information-carrying molecules, nucleotides and their derivatives also contribute to cellular signaling and energy metabolism. The best-known and most-studied examples are derivatives of purine nucleotides, such as ATP, cAMP, cGMP, coenzyme A, FAD, and NAD molecules (1). The balance between purine and pyrimidine nucleotides is critical for A-T and G-C base pairing in DNA synthesis (1). To maintain cellular homeostasis, it is plausible that derivatives of pyrimidine nucleotides also exert regulatory roles on cellular signaling and energy metabolism. However, the roles of pyrimidine nucleotide derivatives in cellular function are less clearly understood compared with purine nucleotide derivatives (1). Dietary nucleotides have been shown to exert protective effects on the immune and gastrointestinal systems, as well as on tissue energy metabolism (2, 3). Yet the underlying mechanisms are vaguely understood, and nutritional values of dietary nucleotides have yet to be established (2).
Uridine, a pyrimidine nucleoside containing uracil and ribose, has been shown to exert protective effects against drug-induced hepatotoxicity and psychiatric disorders (4–6). However, the mechanism underlying the protective roles of uridine has not been elucidated. The fourth step in de novo pyrimidine biosynthesis is catalyzed by dihydroorotate dehydrogenase (DHODH), a mitochondrial membrane-bound and respiratory chain-coupled enzyme (7). Hence, uridine is thought to modulate the mitochondrial respiratory chain through DHODH. Mitochondrial dysfunction is implicated in many human diseases, from metabolic diseases to neurological disorders (8). Thus, uridine has significant therapeutic potential. Indeed, uridine supplementation has been evaluated in many clinical disorders, where its tolerance in humans at supra-physiological concentration was reported (6, 9, 10).
The concentration of circulating plasma uridine of approximately 2–4 μM is tightly regulated throughout different species and individuals (11–13). Most tissues, with the exception of erythrocytes, kidney and liver, rely on the plasma for the supply of uridine (5). Uridine is essentially cleared in a single pass through the liver and is replaced in a highly regulated manner by new uridine formed by de novo synthesis (14). It is plausible that continuous uridine degradation and formation in the liver is necessary for homeostatic control of plasma uridine concentration. Hence, the liver is a suitable tissue for the evaluation of uridine homeostasis and its impact on hepatic cellular function.
Previously, we reported that uridine homeostasis is regulated by uridine phosphorylase 1 (UPase1, encoded by UPP1 gene), an enzyme that catalyzes the reversible conversion of uridine into uracil (15). UPase−/− mice with genetic deletion of UPP1 gene exhibited a 6-fold increase and a 3-fold increase in the plasma and liver uridine concentration, respectively (15, 16). In this study, we generate a transgenic UPase1-TG mouse strain with genetic knock-in of mouse UPP1 gene. We aim to evaluate the impact of constitutive overexpression of UPase1 on liver uridine homeostasis. We anticipate that our study will shed light on the roles of uridine on hepatic cellular function.
MATERIALS AND METHODS
Animals
Two mice strains were used in our experiments, C57BL/6 mice or wild-type (WT) mice (Jackson Laboratories, Bar Harbor, ME) and UPase1-TG mice generated specifically for this study. All mice used were male at 10–12 weeks of age. All animal studies were performed with the approval of the Nevada Cancer Institute and Desert Research Institute Animal Care and Use Committee. All mice were fed with PicoLab Mouse Diet 20 ground pellets (Cat. No. 5058, LabDiet, Brentwood, MO) that provide 4.6 kcal/gram and consist of 22% protein and 9% fat. The lipid composition of the diet includes cholesterol (200 ppm), linoleic acid (2.32%), linolenic acid (0.21%), arachidonic acid (0.02%), and omega-3 fatty acids (0.32%). The total saturated fatty acids and monounsaturated fatty acids are 2.72% and 2.88%, respectively. For mice receiving supplementation, uridine was thoroughly mixed with ground pellets. Approximate daily dosages of uridine were 400 mg per kg body weight per day. Mice received uridine supplementation for five days prior to terminal collection of liver samples for analysis.
UPase1-TG mice generation
We generated a conditional UPase1-TG mouse model targeted at the ROSA26 chromosomal locus (supplementary Fig. IA) (17). The targeting construct contains the UPase1 coding sequence driven by the CAGGS promoter, a hybrid chicken β-actin and cytomegalovirus promoter that was active in almost all tissues in vivo (18). The promoter and UPase1 coding sequence were interposed by a neomycin resistance cassette, which was flanked by loxP sites. Pronuclear microinjection of the transgene construct and subsequent screening was performed by the Animal Genomics Services Core at Yale University School of Medicine. Transgene insertion at the ROSA26 locus was confirmed by Southern blot analysis (supplementary Fig. IB). Gene expression “knock-in” was achieved by crossing these transgenic mice with mice expressing Cre-recombinase in the desired tissue, which excised the loxP sites and released a neomycin resistance cassette allowing CAGGS-driven expression of UPase1. These mice were crossed with FVB/N-Tg(ACTB-cre)2Mrt/J mice (Jackson Laboratories), in which Cre expression was driven by the ubiquitous β-actin promoter.
UPase expression and activity measurement
Expression of UPase1 was analyzed with Western blot analysis using a polyclonal antibody developed in our lab (supplementary Fig. II). UPase activity was measured by the conversion of [3H] uridine to [3H] uracil using high-performance liquid chromatography (HPLC) and scintillation counting. Both methods have been described previously (19).
Liver uridine and β-alanine measurement
Liver uridine and β-alanine were determined using HPLC as described previously (20).
Liver sample preparation for CARS imaging
Liver was perfused with phosphate buffered saline prior to collection. Then, whole liver tissues were sliced with an oscillating tissue slicer (EMS 4500, Electron Microscopy Sciences, Hatfield, PA) into 200 micron thick sections. Liver tissue sections were transferred into glass-bottom, chambered slides and examined with coherent anti-Stokes Raman scattering (CARS) microscopy.
Measurement of liver free fatty acids and triacylglycerides
Liver samples of equal weight (∼40 mg) from each animal group were used for chloroform/methanol total lipid extraction. The recovered organic phases containing lipid were dried and reconstituted in equal volumes (∼100 µl) of chloroform/methanol. Liver triacylglyceride (TAG) levels for nine mice per animal group were determined using the Triacylglyceride Quantification Kit (Cat. No. 10010303, Cayman Chemical, Ann Arbor, MI) and normalized with liver tissue weight. In addition, approximately 50 µl of each liver lipid sample (three mice per animal group) was used for evaluation with LC-MS as previously described (21).
Primary hepatocyte isolation and culturing
Mouse hepatocytes were isolated using a two-step collagenase perfusion technique described previously (22). Culture medium consisted of DMEM/F12 supplemented with 10% FBS, 20 ng/ml epidermal growth factor, 40 ng/ml dexamethasone, 1× insulin/transferrin/selenium supplement, and MycoZap Plus-PR antibiotic mixture. Viability ranged from 70–80% as determined by trypan blue exclusion. Hepatocyte enrichment 4 h after plating reached approximately 90–95%. At 6 h after the initial isolation, hepatocytes were treated with 100 μM uridine for 24 h at 37°C and 5% CO2.
CARS microscopy
The experimental setup of a home-built CARS microscope has been described previously (23). The vibrational frequency used for lipid imaging was fixed at 2,851 cm−1. Images were acquired at approximately 1 s per frame. Approximately 20 and 31 frames were taken along the vertical axis at 1 μm intervals for volumetric imaging of primary hepatocytes and liver tissues, respectively. Liver lipid level measurement based on CARS signal intensity has been described in detail previously (24). Briefly, after subtraction of nonresonant signal and signal arising from cellular membrane, the square root of resonant signal intensity yields a numerical value for the liver lipid level (25, 26). The liver lipid level was normalized to 1 for wild-type, untreated mice and respectively for UPase1-TG mice. Quantitative analysis was performed using the National Institutes of Health ImageJ software.
Screening for the effects of inhibitors and intermediates of pyrimidine biosynthesis pathway on lipid phenotype of primary hepatocytes
Inhibitors and intermediates were added to cultures of primary hepatocytes at 6 h post isolation and density of 2 × 105 cells per ml and incubated for 24 h at 37°C and 5% CO2. The final concentration of inhibitors were 50 nM for brequinar, N-(phosphonacetyl)-l-aspartate (PALA), pyrazofurin (PYR), 5-benzylacyclouridine (BAU), and 5-(2-bromovinyl)uracil (BVU). The final concentrations of intermediates were 10 μM for orotate and 100 μM for uridine and uracil. Hepatocytes were examined with CARS microscopy to evaluate the presence of intracellular lipid accumulation.
DHODH enzymatic assay
The cloning and expression of recombinant dihydroorotate dehydrogenase (DHODH) were described previously (27). The DHODH enzymatic assay was performed in 100 mM HEPES at pH 8.0, 150 mM NaCl, 10% glycerol, 0.05% Triton X-100, 20 μM CoQ0 (Cat. No. D9150, Sigma-Aldrich, St. Louis, MO), 200 μM L-dihydroorotate or DHO (Cat. No. D7128, Sigma-Aldrich), 120 μM 2,6-dichloroindophenol or DCIP (Cat. No. 33125, Sigma-Aldrich) (28). Final DHODH enzyme concentration in each reaction was 50 nM. At 30°C, the oxidation of DHO by DHODH was coupled to the reduction of the chromogen and electron acceptor DCIP. Therefore, DHODH enzymatic activity is measured as a function of the loss of A600 nm of DCIP due to its reduction. DHODH enzymatic activity was determined using the initial linear range.
Measurement of NAD+/NADH and NADP+/NADPH ratios
NAD+/NADH and NADP+/NADPH ratios were measured from liver tissue lysates using commercially available kits according to manufacturer's protocols (Cat. Nos. K337-100 and K347-100, BioVision, Milpitas, CA). The liver tissues from at least six mice per animal group were used for measurement.
Fatty acid β-oxidation measurement
Rates of β-oxidation in primary hepatocytes were measured following previously published methodology (29). Briefly, 50 μCi of [9,10(n)-3H]palmitic acid was added to 2.2 mM unlabeled palmitic acid and evaporated to dryness. The fatty acid was resuspended in 300 μl Krebs-Ringer buffer containing 10 mg/ml BSA at 37°C for 30 min. The reaction mixture was diluted with Krebs-Ringer buffer to a final concentration of 110 μM palmitic acid and specific radioactivity of 5–7E4 cpm/nmol. Primary hepatocytes were plated in 24-well dishes at 70% confluency. After overnight incubation, the monolayers were washed with PBS and then incubated with 200 μl of the palmitic-acid reaction mixture. For inactivated controls, cells were treated with 200 μl absolute methanol for 1 min before the addition of the reaction mixture. The cultures were incubated at 37°C for 2 h. The reaction mixture was removed, and the cells washed twice with 200 μl of PBS. The final combined reaction mixture and washes were transferred to AG1-8× columns converted to the hydroxide form to separate 3H2O from the unreacted substrate. 3H2O was eluted twice with 1 ml of deionized water and collected in scintillation counting vials. Scintillant (10 ml) was added to each milliliter of eluant, and the samples were counted with a Beckman LS6500 scintillation counter. The reaction rate was expressed as nmol 3H2O/h/mg protein. All experiments were performed in triplicate.
1-D Western blots
Total-cell extracts and mitochondrial fractions of the liver tissues were analyzed on 10% SDS-polyacrylamide gel and transferred onto nitrocellulose membrane (Cat. No. BA83, Whatman, Piscataway, NJ). Transferred membrane was blocked with 5% milk and 0.1% Tween-TBS. Blocked membrane was incubated with primary antibodies at 4°C overnight, washed thoroughly, and then incubated with secondary antibodies. Membrane was developed with Enhanced Chemiluminescence (ECL) Reagents (Cat. No. 34075, Thermo Scientific, Rockford, IL). Mitochondrial fractions were collected using the commercially available mitochondria isolation kit for tissue according to manufacturer's protocol (Cat. No. ab110168, Abcam, Cambridge, MA). Primary antibodies were anti-acetylated lysine antibody, Sirt1 antibody, Sirt3 antibody, β-actin antibody (Cat. Nos. 9441, 2028, 5490, and 4967, Cell Signaling, Danvers, MA) and MnSOD antibody (Cat. No. 06-984, Millipore, Billeria, MA). Secondary antibodies were ImmunoPure goat anti-rabbit IgG (H+L) peroxidase conjugated antibody (Cat. No. 31460, Pierce Biotechonology, Rockford, IL).
2-D Western blots and protein identification with MALDI-TOF-MS
Both 2-D Western blots and protein identification were performed by Applied Biomics (Hayward, CA). Briefly, 150 µg of protein from each liver tissue were loaded for 2-D gel electrophoresis, with the first dimension on immobilized pH gradient strips for isoelectric focusing and the second dimension on SDS-PAGE gels. 2-D gels were transferred to PVDF membrane and probed with primary anti-acetylated lysine antibody (rabbit) and secondary Cy3-labeled donkey anti-rabbit IgG antibody. Immuno-positive acetylated protein spots on 2-D Western blots were identified and corresponding spots from duplicate fluorescent dye-labeled 2-D gels were picked automatically with an Ettan Spot Picker. The 2-D gel spots were subjected to in-gel trypsin digestion, peptide extraction, desalting, and then mixed with α-cyano-4-hydroxycinnamic acid (CHCA) matrix and spotted into wells of a MALDI plate for MALDI-TOF-MS identification. Mass spectra of peptides were obtained with an Applied Biosystems proteomics analyzer. Ten of the most abundant peptides in each sample were subjected to fragmentation and further analysis with tandem mass spectrometry (MS/MS). Protein identification was based on peptide fingerprint mass mapping with MS spectra and peptide fragmentation mapping with MS/MS spectra. Database search were performed using the combined MS and MS/MS spectra and the GPS Explorer software equipped with the MASCOT search engine to identify proteins from primary sequence databases. The best match for each 2-D gel spot was presented in the Table 2. As a control, duplicate liver protein samples were also sent to Kendrick Laboratories (Madison, WI) for 2-D Western blots for independent verification of uridine effect on liver protein acetylation profile.
RESULTS
UPase1-TG mice exhibit depletion of endogenous plasma and tissue uridine
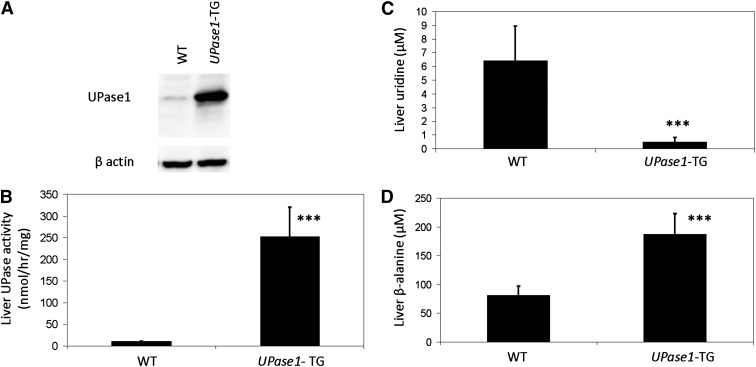
Consistent with our expectation, Western blot analysis revealed an ubiquitous overexpression of UPase1 in the liver tissue (Fig. 1A), as well as in other tissues (supplementary Fig. IIA), of UPase1-TG mice compared with wild-type mice. UPase enzymatic activity in the liver tissue of UPase1-TG mice was 25-fold higher than that of wild-type mice (Fig. 1B). The liver uridine level of UPase1-TG mice was 13-fold lower than that of wild-type mice (Fig. 1C). The liver β-alanine level, which was the product of uridine catabolism, was more than 2-fold higher in UPase1-TG mice compared with wild-type mice (Fig. 1D). Overall, plasma and tissue uridine levels were markedly lower in UPase1-TG mice compared with wild-type mice (Table 1 and supplementary Fig. IIB). Thus, ubiquitous overexpression of UPase1 in UPase1-TG mice caused depletion of endogenous plasma and tissue uridine.
Fig. 1.
A transgenic UPase1 knock-in mouse strain (UPase1-TG) with depleted endogenous uridine level. (A) Elevated UPase1 protein expression in the liver tissues of UPase1-TG mice compared with wild-type mice was observed with Western blot analysis. (B–D) Compared with wild-type mice, UPase1-TG mice exhibited (B) higher liver UPase enzymatic activity, (C) lower liver uridine concentration, and (D) higher liver β-alanine concentration. Error bars are standard deviations of measurements of three mice per animal group. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT control. P values were calculated using Excel's paired Student t-test function.
TABLE 1.
Plasma and tissue uridine concentration of wild-type and UPase1-TG mice
| Plasma Uridine (μM) | Tissue Uridine (μM) |
||||
| Animal Group | Plasma | Gut | Kidney | Liver | Spleen |
| Wild-type | 1.53 ± 0.58 | 29.26 ± 3.38 | 24.56 ± 3.58 | 6.43 ± 2.5 | 4.94 ± 1.13 |
| UPase1-TG | 0.08 ± 0.02 | 9.06 ± 1.50 | 7.08 ± 2.71 | 0.50 ± 0.31 | 0.66 ± 0.25 |
Errors are standard deviations across three mice measured per animal group.
UPase1-TG mice exhibit wild-type blood chemistry, mitochondrial biogenesis and fatty acid metabolism gene expression, and mitochondrial morphology and membrane potential
Despite the depletion of endogenous uridine level, UPase1-TG mice exhibited wild-type characteristics on many biochemical and physiological benchmarks evaluated. In addition to wild-type growth rate and body phenotype, clinical pathology evaluation revealed that UPase1-TG mice exhibited wild-type blood chemistry (supplementary Fig. IIIA) and hematology (data not shown). There was no statistically significant difference between the liver weights of wild-type mice and UPase1-TG mice (data not shown). Gene expression profiling of 84 genes encoding for mitochondrial biogenesis and 84 genes in fatty acid metabolism of the liver tissues revealed no statistically significant difference in expression level of UPase1-TG mice compared with wild-type mice (supplementary Fig. IIIB, C). Furthermore, we found no difference in the liver mitochondrial morphology (supplementary Fig. IIID) or membrane potential (supplementary Fig. IIIE) between wild-type and UPase1-TG mice.
UPase1-TG mice exhibit hepatic microvesicular steatosis
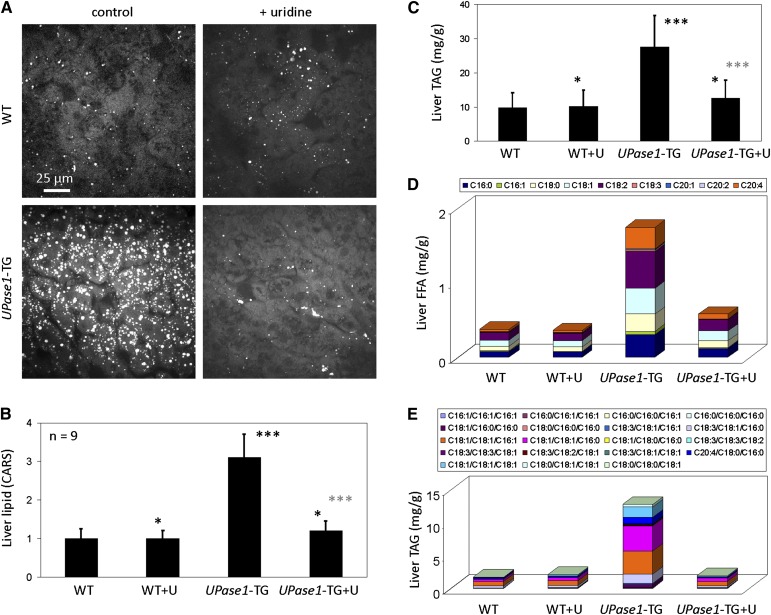
Next, we applied coherent CARS microscopy, a label-free vibrational imaging technique highly sensitive to the detection of hepatic microvesicular steatosis (24), to examine the relationship between uridine homeostasis and liver lipid metabolism. Surprisingly, CARS imaging revealed that the livers of UPase1-TG mice exhibited microvesicular steatosis (Fig. 2A). Quantitative analysis with CARS microscopy revealed that UPase1-TG mice had three times the liver lipid level than wild-type mice (Fig. 2B). Dietary supplementation with 400 mg/kg/day of uridine suppressed hepatic microvesicular steatosis of UPase1-TG mice (Fig. 2A, B). Thus, it is likely that endogenous uridine depletion in UPase1-TG mice was the cause of hepatic microvesicular steatosis. In contrast, wild-type mice fed with 400 mg/kg/day of uridine exhibit wild-type liver lipid phenotype (Fig. 2A, B). Thus, high uridine level due to exogenous dietary supplementation was well-tolerated by the liver. CARS measurement of liver lipid phenotype concurred with established methods of lipid measurement using biochemical and LC-MS assays (Fig. 2C–E). The difference in magnitude between measurement methods was likely due to different interrogation approaches, in which CARS probed for C-H vibration, TAG biochemical measurement probed for TAG hydrolysis, and LC-MS probed for specific FFA or TAG species.
Fig. 2.
UPase1-TG mice exhibit hepatic microvesicular steatosis that can be suppressed with uridine supplementation. (A) CARS imaging reveals that UPase1-TG mice exhibit intrinsic hepatic microvesicular steatosis, which can be suppressed with dietary uridine supplementation at 400 mg/kg/day. (B) Quantitative analysis of liver lipid level with CARS microscopy. (C) Liver triacylglyceride level determined with biochemical assays. Error bars in (A) and (B) represent standard deviation across nine mice analyzed per animal or treatment group. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT control (black asterisks) or UPase1-TG control (gray asterisks). P values were calculated using Excel's paired Student t-test function. (D, E) LC-MS evaluation of liver. (D) Free fatty acids (FFA) composition and (E) triacylglyceride (TAG). LC-MS data are average of three mice per animal or treatment group.
Uridine suppresses hepatic microvesicular steatosis in UPase1-TG mice with an EC50 value of approximately 20 μM
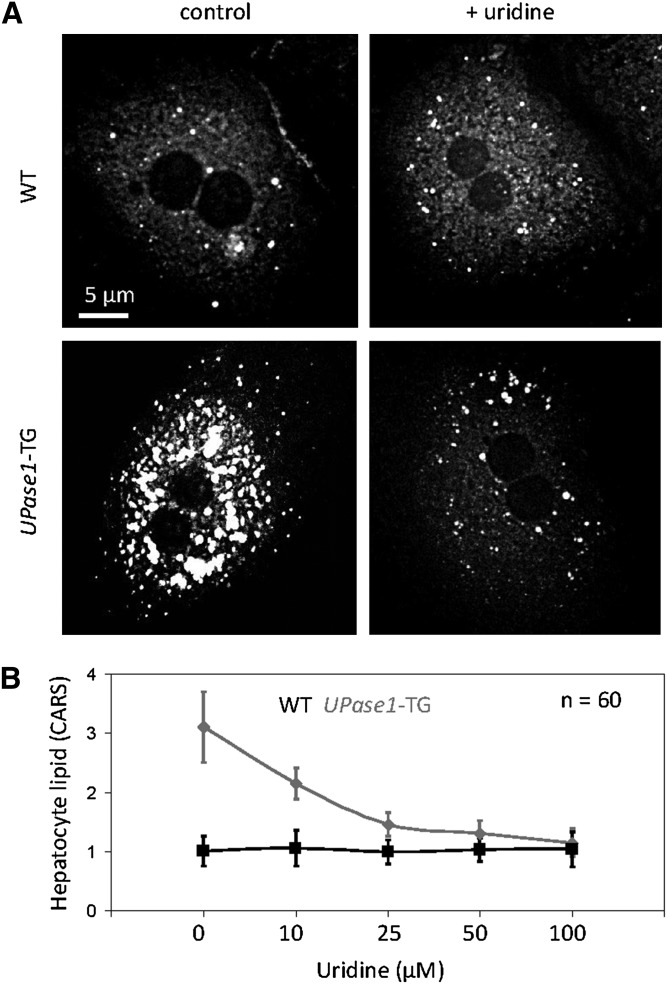
Next, we evaluated the effective concentration of exogenous uridine for the suppression of microvesicular steatosis associated with the UPase1-TG genotype in primary hepatocytes. CARS imaging revealed that primary hepatocytes of both wild-type and UPase1-TG mice faithfully reproduced their in vivo phenotypes (Fig. 3A). UPase1-TG hepatocytes exhibited a dose-dependent response to exogenous uridine supplementation. The concentration of uridine that caused a 50% suppression of microvesicular steatosis of UPase1-TG hepatocytes after 24 h or EC50 was approximately 20 μM (Fig. 3B). Complete suppression of microvesicular steatosis of UPase1-TG hepatocytes was achieved with exogenous uridine concentration of 50–100 μM (Fig. 3B). The effective uridine dosage observed in our experiment was consistent with a previous report on the dose response of uridine to reverse the adverse effects by nucleoside reverse transcriptase inhibitors (NRTI) on adipocyte function (30). In contrast, wild-type hepatocytes exhibited no change to lipid phenotype in the absence or presence of uridine supplementation (Fig. 3A, B).
Fig. 3.
Determination of EC50 value for uridine to suppress microvesicular steatosis. (A) Primary hepatocytes of wild-type and UPase1-TG faithfully reproduce their in vivo phenotypes. (B) Dose-dependent effect of uridine supplementation for the suppression of microvesicular steatosis in UPase1-TG primary hepatocytes. Error bars are standard deviations for 60 hepatocytes analyzed per animal or treatment group.
Uridine suppresses brequinar- and orotate-induced steatosis in primary hepatocytes
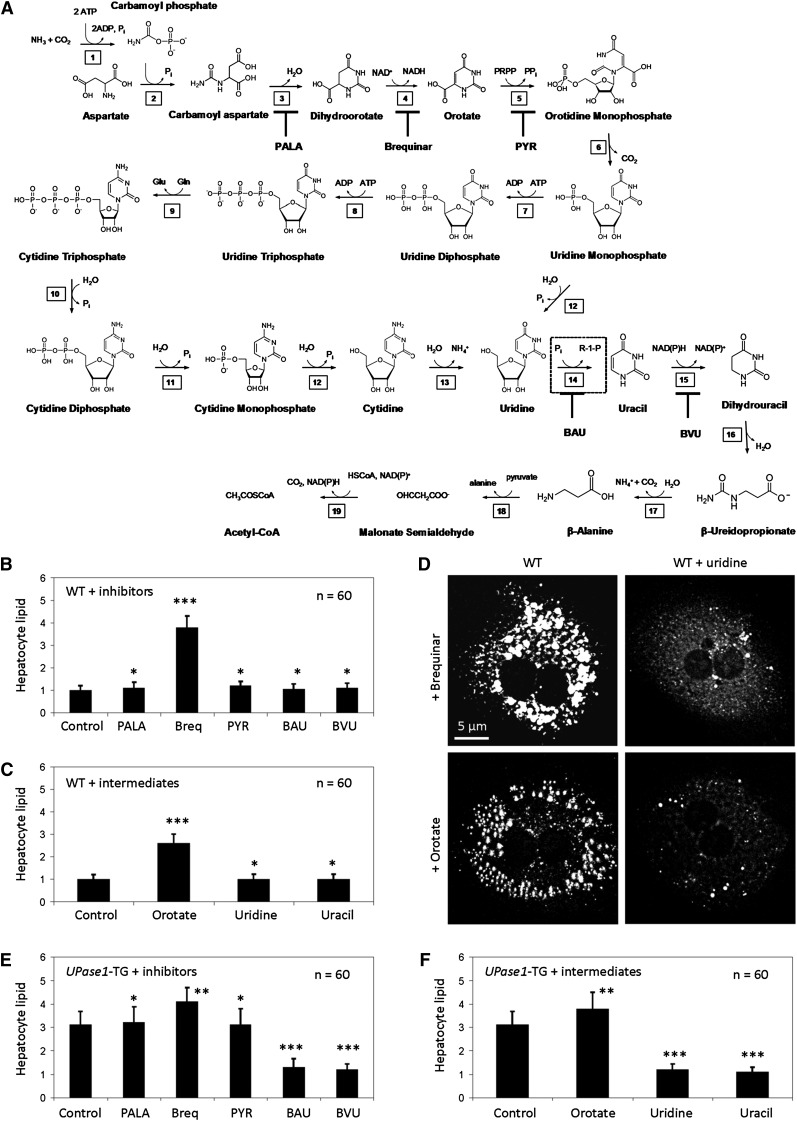
To identify the potential cause of liver lipid phenotype, we screened for the effects of inhibitors and intermediates of the pyrimidine biosynthesis pathway (Fig. 4A) on wild-type primary hepatocytes with CARS microscopy. We found that among the inhibitors evaluated, only the addition of brequinar, an allosteric inhibitor of DHODH, induced intracellular lipid accumulation (Fig. 4B). On the other hand, of the intermediates evaluated, only the addition of orotate, an end product of DHODH enzymatic activity, induced intracellular lipid accumulation (Fig. 4C). Our data suggested that either allosteric or end-product inhibition of DHODH was sufficient to induce lipid phenotype of primary hepatocytes. DHODH catalyzes the only mitochondrial step in nucleotide rings biosynthesis. DHODH also represents the only step of all anabolic pathways of nucleotide and amino acid biosynthesis that depends strictly on the presence of oxygen (7). It is widely believed that respiratory chain-coupled DHODH links mitochondrial energy metabolism with pyrimidine biosynthesis (7, 31). Thus, it is plausible that brequinar or orotate induced lipid phenotype of primary hepatocytes through the inhibition of DHODH and, subsequently, mitochondrial respiration. Interestingly, uridine supplementation reversed both brequinar- and orotate-induced lipid phenotype in cultured primary hepatocytes (Fig. 4D).
Fig. 4.
Inhibition of DHDOH induces steatosis, and inhibition of uridine catabolism suppresses steatosis. (A) Pyrimidine biosynthesis and catabolism pathway. 1) Carbamoyl phosphate synthetase; 2) Aspartic transcarbamoylase; 3) Dihydroorotase; 4) Dihydroorotate dehydrogenase (DHODH); 5) Orotate phosphoribosyltransferase; 6) Orotidine 5′-phosphate decarboxylase; 7) Uridine-cytidine kinase 2; 8) Nucleoside-diphosphate kinase; 9) Cytidine triphosphate synthase; 10) Nucleoside triphosphatase; 11) Nucleoside diphosphatase; 12) Nucleotidase; 13) Cytidine deaminase; 14) Uridine phosphorylase (UPase, dashed box); 15) Dihydropyrimidine dehydrogenase (DPD); 16) Hydropyrimidine hydratase (also known as dihydropyrimidinase); 17) β-Ureidopropionase; 18) 3-Alanine-pyruvate transaminase; 19) Malonate semialdehyde dehydrogenase. Inhibitors of the pyrimidine biosynthesis and catabolism pathway are N-(phosphonacetyl)-l-aspartate (PALA), brequinar, pyrazofurin (PYR), 5-benzylacyclouridine (BAU), and 5-(2-bromovinyl)uracil (BVU). (B) Brequinar, an allosteric inhibitor of DHODH, and (C) orotate, an enzymatic product of DHODH, induce lipid phenotype in wild-type hepatocytes. Error bars are standard deviations of 60 hepatocytes analyzed per experimental condition. (D) Uridine supplementation suppresses lipid phenotype induced by brequinar and orotate. (E) BAU and BVU suppress lipid phenotype of UPase1-TG hepatocytes. (F) Uridine and uracil supplementation suppress lipid phenotype of UPase1-TG hepatocytes. Error bars are standard deviations of 60 hepatocytes analyzed per animal group or treatment condition. *P < 0.05, **P < 0.01, ***P < 0.001 versus control. P values were calculated using Excel's paired Student t-test function.
Inhibition of uridine catabolism suppresses steatosis in UPase1-TG primary hepatocytes
Similarly, we screened for the effect of inhibitors and intermediates of the pyrimidine biosynthesis pathway on UPase1-TG primary hepatocytes with CARS microscopy. Of the inhibitors evaluated, brequinar aggravated the lipid phenotype of UPase1-TG primary hepatocytes (Fig. 4E). Benzylacyclouridine (BAU), an inhibitor of UPase enzymatic activity, and bromovinyl uracil (BVU), an inhibitor of dihydropyrimidine dehydrogenase (DPD), which is an enzyme immediately downstream of UPase in the uridine catabolism pathway, suppressed the lipid phenotype of UPase1-TG primary hepatocytes (Fig. 4E). Of the intermediates evaluated, orotate aggravated the lipid phenotype of UPase1-TG primary hepatocytes (Fig. 4F). Uridine and uracil suppressed the lipid phenotype of UPase1-TG primary hepatocytes (Fig. 4F). It is necessary to point out that UPase1 catalyzes a reversible conversion of uridine to uracil (32). Hence, an increase in uracil concentration would also lead to an increase in uridine. Our data revealed that inhibition of DHODH aggravated lipid phenotype of UPase1-TG primary hepatocytes. In contrast, inhibitors or intermediates of the pyrimidine biosynthesis pathway that conserved or elevated uridine concentration suppressed the lipid phenotype of UPase1-TG primary hepatocytes.
Uridine has no effect on in vitro DHODH enzymatic activity
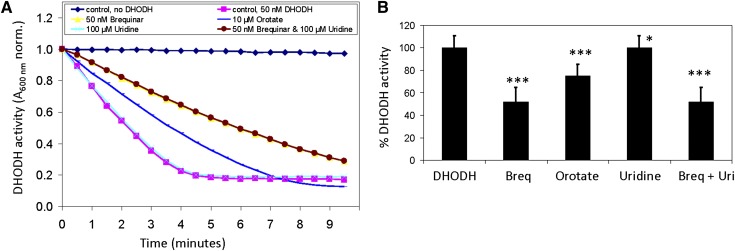
To determine whether uridine reversed the lipid phenotype of hepatocytes by acting directly on DHODH, we evaluated in vitro the enzymatic activity of purified recombinant DHODH in the presence or absence of uridine. In vitro DHODH enzymatic activity was determined indirectly through the disappearance of absorbance at 600 nm wavelength due to the reduction of the chromogen 2,6-dichloroindophenol (DCIP) (27, 33). We found that the addition of 50 nM brequinar and 10 μM orotate suppressed DHODH enzymatic activity by 50% and 25%, respectively (Fig. 5A, B). In contrast, addition of 100 μM uridine had no impact on DHODH enzymatic activity. Although uridine supplementation suppressed the brequinar-induced lipid phenotype in primary hepatocytes (Fig. 4D), the addition of uridine did not reverse the inhibition of DHODH enzymatic activity by brequinar (Fig. 5A, B). Our data suggest that uridine did not exert its protective effect against hepatic microvesicular steatosis by acting directly on DHODH.
Fig. 5.
Brequinar and orotate inhibit whereas uridine has no effect on in vitro DHODH enzymatic activity. (A) Representative enzymatic kinetics of purified recombinant DHODH in the presence and absence of brequinar, orotate, and uridine. (B) Quantitative analysis of DHODH enzymatic activity in the presence and absence of brequinar, orotate, and uridine. Error bars are standard deviations of triplicate experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus DHODH control. P values were calculated using Excel's paired Student t-test function.
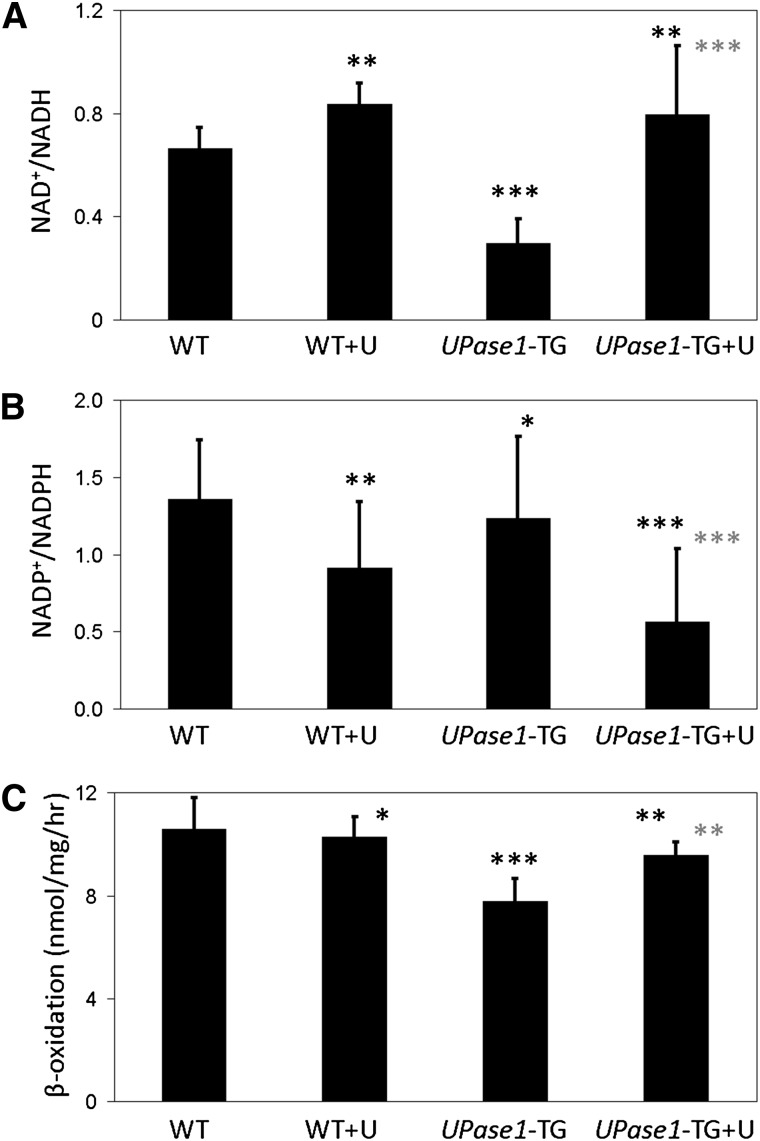
Uridine induces changes to liver NAD+/NADH and NADP+/NADPH ratios and rates of fatty acid β-oxidation
On the other hand, uridine supplementation induced a number of changes to the molecular profiles of liver tissues and primary hepatocytes. We found that the liver tissues of wild-type and UPase1-TG mice fed with uridine exhibited elevated NAD+/NADH ratio compared with untreated control mice (Fig. 6A). In contrast, the NADP+/NADPH ratio was reduced in the liver tissues of both wild-type and UPase1-TG mice fed with uridine compared with untreated control mice (Fig. 6B). In isolated primary hepatocytes, the fatty acid β-oxidation rate of UPase1-TG hepatocytes was approximately 25% lower than wild-type hepatocytes (Fig. 6C). Uridine supplementation partially restored the fatty acid β-oxidation rate of UPase1-TG hepatocytes, whereas it had no significant impact on the fatty acid β-oxidation rate of wild-type hepatocytes (Fig. 6C).
Fig. 6.
Uridine induces changes to liver NAD+/NADH and NADP+/NADPH ratios and rates of fatty acid β-oxidation. Uridine supplementation at 400 mg/kg/day causes (A) increases of the NAD+/NADH ratio and (B) decreases of the NADP+/NADPH ratio in the livers of both wild-type and UPase1-TG mice. Error bars represent standard deviation across six mice analyzed per animal or treatment group. (C) Uridine supplementation at 100 μM increases the fatty acid β-oxidation rate of the UPase1-TG primary hepatocytes while having no impact on the fatty acid β-oxidation rate of the wild-type primary hepatocytes. Error bars are standard deviations of triplicate measurements. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT control (black asterisks) or UPase1-TG control (gray asterisks). P values were calculated using Excel's paired Student t-test function.
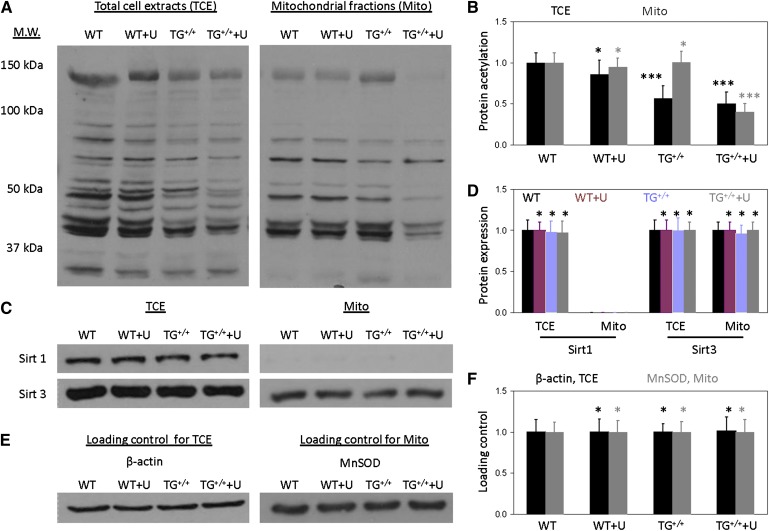
1-D Western blots reveal reduced liver protein acetylation profile of UPase1-TG mice due to uridine supplementation
Changes to the NAD+/NADH ratio due to uridine supplementation would likely affect the activity of NAD+-dependent deacetylases. Thus, we evaluated the liver protein acetylation profile with 1-D Western blots using antibodies against acetylated lysine residues. Consistent with many previous reports (34–36), we observed that mitochondrial fractions contributed significantly to the overall liver protein acetylation profile (Fig. 7A, B). Uridine supplementation had no observable impact on the liver protein acetylation profile of wild-type mice in either total-cell extracts or mitochondrial fractions. In contrast, uridine supplementation caused a reduction in the liver protein acetylation profile of UPase1-TG mice in both total-cell extracts and mitochondrial fractions (Fig. 7A, B). Because protein acetylation is regulated by a family of NAD+-regulated deacetylases (37), we evaluated the protein expression of Sirt1 and Sirt3 with 1-D Western blots (Fig. 7C, D). Sirt1 was found only in the total-cell extracts and not in the mitochondrial fractions; whereas, Sirt3 was found in both total-cell extracts and mitochondrial fractions. Both Sirt1 and Sirt3 protein expression level were unaffected by uridine supplementation in either wild-type or UPase1-TG mice (Fig. 7C–F). Our data suggest that decreases in liver protein acetylation profile in UPase1-TG mice treated with uridine are due to changes in the enzymatic activities of Sirt1 and Sirt3 (supplementary Fig. IV) and not their expression level.
Fig. 7.
1-D Western blot reveals uridine-induced changes to the liver protein acetylation profile of UPase1-TG mice. Western blot lane assignment: WT, wild-type; WT+U, wild-type + uridine; TG+/+, UPase1-TG; TG+/++U, UPase1-TG + uridine. (A) Protein lysine acetylation profiles of total-cell extracts (TCE) and mitochondrial fractions (Mito). (B) Quantitative analysis of protein lysine acetylation profiles presented in (A). (C) Sirt1 and Sirt3 protein expression level of TCE and Mito. (D) Quantitative analysis of Sirt1 and Sirt3 protein expression level presented in (C). (E) Loading controls for TCE and Mito samples presented in both (A) and (C). β-actin and mitochondrial manganese superoxide dismutase (MnSOD) were used as loading controls for TCE and Mito, respectively. All data presented are representative of three mice per animal or treatment group used for Western blot analysis. (F) Quantitative analysis of loading controls presented in (E). Data are normalized to 1 for WT control and respectively, for UPase1-TG. Error bars are standard deviations across three mice per animal group evaluated. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT control (black asterisks, TCE; gray asterisks, Mito). P values were calculated using Excel’s paired Student t-test function.
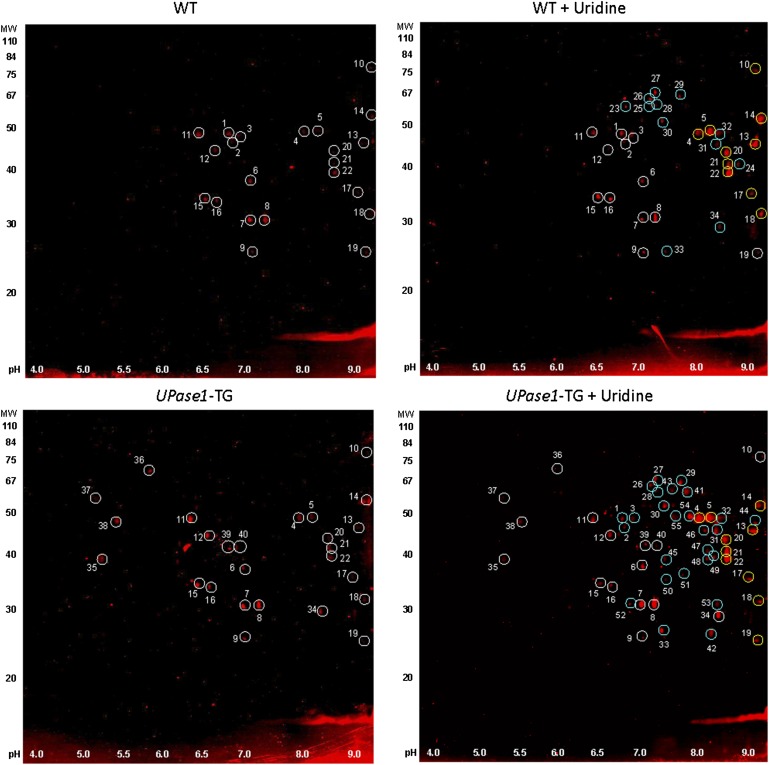
2-D Western blots reveal changes to hepatic protein acetylation profile due to uridine supplementation
We further evaluated the protein acetylation profiles of liver total-cell extracts with 2-D Western blots using antibodies against acetylated lysine residues. 2-D Western blots revealed that uridine supplementation induced significant changes to the liver protein acetylation profile in both wild-type and UPase1-TG mice (Fig. 8 and supplementary Figs. V and VI). For wild-type mice fed a control diet, 22 protein spots were identified that were positively immuno-labeled with antibodies against acetylated lysine (Fig. 8, white circles). Wild-type mice fed with a uridine-supplemented diet exhibited 34 immuno-positive spots, or 12 more new spots, compared with wild-type mice fed a control diet (Fig. 8, cyan circles). On the other hand, UPase1-TG mice fed a control diet exhibited 26 immuno-positive spots (Fig. 8, white circles). UPase1-TG mice fed with a uridine-supplemented diet exhibited 52 immuno-positive spots, or 26 more new spots, compared with UPase1-TG mice fed with a control diet (Fig. 8, cyan circles). In addition, 10 immuno-positive spots exhibited increases in signal intensity due to uridine supplementation in both wild-type and UPase1-TG mice (Fig. 8, yellow circles).
Fig. 8.
2-D Western blot reveals potential targets of uridine-induced protein acetylation. Circled spots were picked for protein identification with MALDI-MS for (A) WT control (upper left panel) and WT + uridine (upper right panel) and (B) UPase1-TG (lower left panel) and UPase1-TG + uridine (lower right panel). White circles are immuno-positive spots unaffected by uridine supplementation. Yellow circles are immuno-positive spots whose intensity increases due to uridine supplementation. Cyan circles are new immuno-positive spots induced by uridine supplementation.
Identification of acetylated proteins with MALDI-TOF-MS
To identify the acetylated proteins affected by uridine supplementation, the immuno-positive spots were picked and identified with MALDI-TOF-MS). Of the total 55 immuno-positive spots, 42 unique proteins were identified (Table 2 and supplementary Table I). Many proteins were present in multiple spots due to possible posttranslational modifications. Eight immuno-positive spots containing 7 specific proteins were acetylated due to uridine supplementation in both wild-type and UPase1-TG mice (Table 2, highlighted box). These 7 proteins participate in antioxidation (catalase and superoxide dismutase), urea/tricarboxylic cycle (delta-1-pyrroline-5-carboxylate dehydrogenase), pyrimidine metabolism (methylmalonate-semialdehyde dehydrogenase), cholesterol/steroid metabolism (hydroxymethylglutaryl-CoA synthase), oxidation-reduction (alcohol dehydrogenase 1), and amino acid metabolism (betaine homocysteine S-methyltransferase 1). In addition, 9 specific proteins were identified out of the 10 immuno-positive spots that exhibited increased signal intensity due to uridine supplementation in both wild-type and UPase1-TG mice. Eight of these 9 proteins participate in amino acid metabolism (argininosuccinate synthase), lipid metabolism (3-ketoacyl-CoA thiolase B, peroxisomal acyl-CoA oxidase 1), protein translation (elongation factor 1-α 1), ketone body metabolism (D-β-hydroxybutyrate dehydrogenase), glycolysis (fructose-bisphosphate aldolase B, glyceraldehyde-3-phosphate dehydrogenase), and purine metabolism (uricase). The function of the other protein, Protein NipSnap homolog 1, is currently unknown. The acetylated proteins reported in this work were also identified in previous comprehensive liver acetylome analyses (38–40). Although we identified only 42 acetylated proteins in this study, previous studies have identified as many as 1,047 acetylated proteins in human liver (38) and 195 acetylated proteins from mouse liver (39). Our 2-D Western blots revealed that uridine supplementation increased the acetylation of the identified proteins in both wild-type and UPase1-TG mice. In contrast, 1-D Western blots showed that uridine supplementation reduced the total protein acetylation profile of UPase1-TG mice. It is possible that while some proteins were acetylated, other proteins were deacetylated due to uridine supplementation. Increased NAD+/NADH ratio with uridine supplementation (Fig. 6A) is likely to activate NAD+-regulated deacetylases, leading to protein deacetylation. On the other hand, uridine catabolism can lead to increases of the cellular acetyl-CoA pool, leading to increased protein acetylation (Fig. 4A). The precise effect of uridine on protein acetylation or deacetylation is unclear. Nonetheless, our proteomic data reveal that uridine supplementation modifies the acetylation profile of metabolic, oxidation-reduction, and antioxidation enzymes.
TABLE 2.
Uridine induces changes to the protein acetylation profile
| Spot Number | Protein Name | WT | WT + Uridine | UPase1-TG | UPase1-TG + Uridine | Biological Process |
| 1 | SEC14-like protein 2 | √ | √ | — | √ | Cellular transport |
| 2 | Fumarylacetoacetase | √ | √ | — | √ | Amino acid metabolism |
| 3 | Isocitrate dehydrogenase [NADP] | √ | √ | — | √ | TCA cycle |
| 4 | Argininosuccinate synthase | √ | √√ | √ | √√ | Amino acid metabolism |
| 5 | Argininosuccinate synthase | √ | √√ | √ | √√ | Amino acid metabolism |
| 6 | Glycine N-methyltransferase | √ | √ | √ | √ | Amino acid metabolism |
| 7 | Carbonic anhydrase 3 | √ | √ | √ | √ | Acid-base balance |
| 8 | Carbonic anhydrase 3 | √ | √ | √ | √ | Acid-base balance |
| 9 | Superoxide dismutase [Mn] | √ | √ | √ | √ | Antioxidation |
| 10 | Peroxisomal bifunctional enzyme | √ | √√ | √ | √ | Lipid metabolism |
| 11 | Acyl-CoA thioesterase 1 | √ | √ | √ | √ | Lipid metabolism |
| 12 | Arginase-1 | √ | √ | √ | √ | Urea cycle |
| 13 | 3-ketoacyl-CoA thiolase B | √ | √√ | √ | √√ | Lipid metabolism |
| 14 | Elongation factor 1-α 1 | √ | √√ | √ | √√ | Protein translation |
| 15 | Cytochrome c1, heme protein | √ | √ | √ | √ | Electron transport |
| 16 | L-xylulose reductase | √ | √ | √ | √ | Glucose metabolism |
| 17 | D-β-hydroxybutyrate dehydrogenase | √ | √√ | √ | √√ | Ketone body metabolism |
| 18 | Protein NipSnap homolog 1 | √ | √√ | √ | √√ | Unknown |
| 19 | Peroxisomal acyl-CoA oxidase 1 | √ | √ | √ | √√ | Lipid metabolism |
| 20 | Fructose-bisphosphate aldolase B | √ | √√ | √ | √√ | Glycolysis |
| 21 | Glyceraldehyde-3-phosphate dehydrogenase | √ | √√ | √ | √√ | Glycolysis |
| 22 | Uricase | √ | √√ | √ | √√ | Purine metabolism |
| 23 | Glutamate dehydrogenase 1 | — | √ | — | — | Amino Acid metabolism |
| 24 | Malate dehydrogenase | — | √ | — | — | TCA cycle |
| 25 | Dihydropyrimidinase | — | √ | — | — | Pyrimidine metabolism |
| 26 | Catalase | — | √ | — | √ | Antioxidation |
| 27 | Delta-1-pyrroline-5-carboxylate dehydrogenase | — | √ | — | √ | Urea/TCA cycle |
| 28 | Methylmalonate-semialdehyde dehydrogenase | — | √ | — | √ | Pyrimidine metabolism |
| 29 | Catalase | — | √ | — | √ | Antioxidation |
| 30 | Hydroxymethylglutaryl-CoA synthase | — | √ | — | √ | Steroid metabolism |
| 31 | Alcohol dehydrogenase 1 | — | √ | — | √ | Oxidation-reduction |
| 32 | Betaine homocysteine S-methyltransferase 1 | — | √ | — | √ | Amino acid metabolism |
| 33 | Superoxide dismutase [Mn] | — | √ | — | √ | Antioxidation |
| 34 | Glutathione S-transferase | — | √ | √ | √ | Antioxidation |
| 35 | Regucalcin | — | — | √ | √ | Calcium signaling |
| 36 | Heat shock cognate 71 kDa protein | — | — | √ | √ | Stress response |
| 37 | ATP synthase subunit β | — | — | √ | √ | ATP biosynthesis |
| 38 | Actin, cytoplasmic 2 | — | — | √ | √ | Cytoskeleton |
| 39 | Alcohol dehydrogenase 1 | — | — | √ | √ | Oxidation-reduction |
| 40 | Carbonic anhydrase 3 | — | — | √ | √ | Acid-base balance |
| 41 | Retinal dehydrogenase 1 | — | — | — | √ | Retinol metabolism |
| 42 | Glutathione S-transferase | — | — | — | √ | Antioxidation |
| 43 | Retinal dehydrogenase 1 | — | — | — | √ | Retinol metabolism |
| 44 | Cytochrome b-c1 complex subunit 2 | — | — | — | √ | Electron transport |
| 45 | L-lactate dehydrogenase A | — | — | — | √ | Oxidation-reduction |
| 46 | Alcohol dehydrogenase 1 | — | — | — | √ | Oxidation-reduction |
| 47 | Glyceraldehyde-3-phosphate dehydrogenase | — | — | — | √ | Glycolysis |
| 48 | Uricase | — | — | — | √ | Purine metabolism |
| 49 | Estradiol 17 β-dehydrogenase 5 | — | — | — | √ | Steroid metabolism |
| 50 | Guanine nucleotide-binding protein subunit β-2-like 1 | — | — | — | √ | Cellular signaling |
| 51 | Glycine N-acyltransferase-like protein | — | — | — | √ | Amino acid metabolism |
| 52 | Carbonic anhydrase 3 | — | — | — | √ | Acid-base balance |
| 53 | Electron transfer flavoprotein subunit β | — | — | — | √ | Electron transport |
| 54 | Betaine homocysteine S-methyltransferase 1 | — | — | — | √ | Amino acid metabolism |
| 55 | Argininosuccinate synthase | — | — | — | √ | Amino acid metabolism |
—, absence of acetylated protein; √, presence of acetylated protein; √√, increase in expression of acetylated protein due to uridine supplementation; bold font, proteins with multiple spots on 2-D gel; highlighted box, proteins acetylated due to uridine supplementation in both wild-type and UPase1-TG mice. WT, wild-type.
DISCUSSION
In this study, we generated a transgenic mouse model UPase1-TG with genetic knock-in of UPase1 to study the roles of uridine homeostasis in liver function. Compared with wild-type mice, UPase1-TG mice had severe reduction of plasma and tissue uridine concentration due to elevated expression and activity of UPase1. In addition, UPase1-TG mice exhibited hepatic microvesicular steatosis, which could be suppressed with exogenous uridine supplementation at 400 mg/kg/day. In primary UPase1-TG hepatocytes, the EC50 value for uridine to suppress intracellular lipid accumulation was approximately 20 μM. In contrast, uridine supplementation at 400 mg/kg/day for wild-type mice or at 100 µM for primary hepatocytes did not cause any observable intracellular lipid accumulation. Taken together, our data reveal that uridine is critical for the maintenance of a fat-free liver. High uridine concentration is well-tolerated by the liver in term of lipid phenotype, whereas depletion of uridine causes hepatic microvesicular steatosis.
We found that the pyrimidine biosynthesis and catabolism pathway is linked to fatty liver via two possible mechanisms: i) inhibition of the mitochondrial membrane-bound and respiratory chain-coupled DHODH enzyme and ii) overexpression of the UPase1 enzyme, which causes depletion of endogenous uridine level. Consistent with our observation, orotate was reported to induce fatty liver in rodents more than half a century ago (41), but the precise mechanism has yet to be delineated (42). Our data suggest that orotate causes fatty liver by impairing hepatic mitochondrial respiration via inhibition of DHODH enzymatic activity. Surprisingly, uridine suppressed intracellular lipid accumulation induced by orotate or brequinar, an end-product inhibitor and an allosteric inhibitor of DHODH enzymatic activity, respectively. In fact, uridine has been used as a therapy for orotic aciduria, a hereditary condition characterized by excessive excretion of orotic acid in urine (43). Because uridine did not have any effect on DHODH enzymatic activity, it is likely that uridine reversed orotate-induced hepatic intracellular lipid accumulation via means other than the modulation of DHODH enzymatic activity. On the other hand, restoring uridine concentration in UPase1-TG mice via inhibition of endogenous uridine catabolism or via exogenous uridine supplementation also suppressed liver lipid phenotype. Clearly, uridine is an important molecule in the regulation of liver lipid metabolism.
Interestingly, we found that uridine supplementation induced changes to the liver protein acetylation profile. Most of the proteins, whose acetylation profiles were affected by uridine supplementation, participate in cellular metabolism, oxidation reduction, and antioxidation processes. Consistently, uridine supplementation also induced changes to the NAD+/NADH and NADP+/NADPH ratios, which are coenzymes crucial for cellular metabolism, oxidation reduction, and antioxidation processes. In recent years, evidence in the literature supports the regulation of cellular energy metabolism by protein lysine acetylation (44). Protein lysine acetylation is a prevalent postgenomic modification of nearly all enzymes participating in cellular energy metabolism in the liver tissue (38). Indeed, acetylation is emerging as a general mechanism that controls cellular adaptation to changes in energy metabolism (45). The regulation of metabolic enzymes by acetylation remains to be elucidated (46); nonetheless, it is plausible that uridine modulates liver protein acetylation profile, leading to the suppression of fatty liver.
The precise mechanisms underlying the relationship between uridine homeostasis and fatty liver remain to be elucidated. In this work, we report the effect of uridine on the modulation of liver protein acetylation profile and the suppression of hepatic microvesicular steatosis. A previous study independently reported gene expression regulation of a liver-specific uridine phosphorylase by multiple lipid-sensing nuclear receptors (47). This observation suggests a potential role of the nuclear receptor signaling pathway in linking liver lipid metabolism and uridine homeostasis. When considering the tight regulation of uridine concentration by the liver (14), it is conceivable that uridine homeostasis plays a critical role in liver energy metabolism. The UPase1-TG mouse strain generated for this work and the UPase−/− mouse strain generated previously (15) provide suitable animal models for future in-depth investigation into the relationship between uridine homeostasis and liver health.
Fatty liver is a becoming a prevalent medical condition in the United States due to the obesity epidemic in young children (48), the aging population (49, 50), and the widespread use of prescription drugs (51). Fatty liver is also an established risk factor for fatty liver disease (52). Clinical treatment of fatty liver relies mainly on the management of the underlying medical condition, such as diabetes, obesity, or hyperlipidemia (53). Currently, there is no known direct treatment of fatty liver condition (53). Given the link between uridine homeostasis, pyrimidine metabolism, and liver lipid accumulation, there is a potential for the use of nucleosides and nucleoside analogs in the treatment of fatty liver condition.
Supplementary Material
Acknowledgments
The authors thank Dr. Margaret A. Phillips (University of Texas Southwestern) for the generous gift of the recombinant DHODH vector, Dr. Tarmo Roosild (Nevada Cancer Institute) for the purification of DHODH protein, Dr. Franklin Chin (Applied Biomics) for proteomics services, and Dr. Vera Samburova (Desert Research Institute) for LC/MS analysis of liver lipid.
Footnotes
Abbreviations:
- 1(2)-D
- one (two)-dimensional
- CARS
- coherent anti-Stokes Raman scattering
- DHODH
- dihydroorotate dehydrogenase
- MALDI-TOF-MS
- MALDI time-of-flight MS
- TAG
- triacylglyceride
- UPase
- uridine phosphorylase
- WT
- wild-type
This work was supported by National Institutes of Health Grant P20RR-016464 (to T.T.L.), American Cancer Society Grant IRG-08-062-04 (to T.T.L.), and the Vons Breast Cancer Research Award (to T.T.L. and G.P.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures, and two tables.
REFERENCES
- 1.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P.. 2008. Molecular Biology of the Cell. 5th edition. Garland Science, Taylor & Francis Group, NY. [Google Scholar]
- 2.Carver J. D., Walker W. A. 1995. The role of nucleotides in human nutrition. J. Nutr. Biochem. 6: 58–72. [Google Scholar]
- 3.Cantó C., Houtkooper R. H., Pirinen E., Youn D. Y., Oosterveer M. H., Cen Y., Fernandez-Marcos P. J., Yamamoto H., Andreux P. A., Cettour-Rose P., et al. 2012. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15: 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Setzer B., Lebrecht D., Walker U. A. 2008. Pyrimidine nucleoside depletion sensitizes to the mitochondrial hepatotoxicity of the reverse transcriptase inhibitor stavudine. Am. J. Pathol. 172: 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traut T. W., Jones M. E. 1996. Uracil metabolism-UMP synthesis from orotic acid or uridine and conversion of uracil to beta-alanine: enzymes and cDNAs. Prog. Nucleic Acid Res. Mol. Biol. 53: 1–78. [DOI] [PubMed] [Google Scholar]
- 6.Connolly G. P., Duley J. A. 1999. Uridine and its nucleotides: biological actions, therapeutic potentials. Trends Pharmacol. Sci. 20: 218–225. [DOI] [PubMed] [Google Scholar]
- 7.Löffler M., Jockel J., Schuster G., Becker C. 1997. Dihydroorotat-ubiquinone oxidoreductase links mitochondria in the biosynthesis of pyrimidine nucleotides. Mol. Cell. Biochem. 174: 125–129. [PubMed] [Google Scholar]
- 8.Wallace D. C. 2005. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39: 359–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutinen J., Walker U. A., Sevastianova K., Klinker H., Hakkinen A. M., Ristola M., Yki-Jarvinen H. 2007. Uridine supplementation for the treatment of antiretroviral therapy-associated lipoatrophy: a randomized, double-blind, placebo-controlled trial. Antivir. Ther. 12: 97–105. [PubMed] [Google Scholar]
- 10.Weinberg M. E., Roman M. C., Jacob P., Wen M., Cheung P., Walker U. A., Mulligan K., Schambelan M. 2011. Enhanced uridine bioavailability following administration of a triacetyluridine-rich nutritional supplement. PLoS ONE. 6: e14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizzorno G., Yee L., Burtness B. A., Marsh J. C., Darnowski J. W., Chu M. Y., Chu S. H., Chu E., Leffert J. J., Handschumacher R. E., et al. 1998. Phase I clinical and pharmacological studies of benzylacyclouridine, a uridine phosphorylase inhibitor. Clin. Cancer Res. 4: 1165–1175. [PubMed] [Google Scholar]
- 12.van Groeningen C. J., Leyva A., Kraal I., Peters G. J., Pinedo H. M. 1986. Clinical and pharmacokinetic studies of prolonged administration of high-dose uridine intended for rescue from 5-FU toxicity. Cancer Treat. Rep. 70: 745–750. [PubMed] [Google Scholar]
- 13.Traut T. W. 1994. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140: 1–22. [DOI] [PubMed] [Google Scholar]
- 14.Gasser T., Moyer J. D., Handschumacher R. E. 1981. Novel single-pass exchange of circulating uridine in rat liver. Science. 213: 777–778. [DOI] [PubMed] [Google Scholar]
- 15.Cao D., Leffert J. J., McCabe J., Kim B., Pizzorno G. 2005. Abnormalities in uridine homeostatic regulation and pyrimidine nucleotide metabolism as a consequence of the deletion of the uridine phosphorylase gene. J. Biol. Chem. 280: 21169–21175. [DOI] [PubMed] [Google Scholar]
- 16.Cao D., Ziemba A., McCabe J., Yan R., Wan L., Kim B., Gach M., Flynn S., Pizzorno G. 2011. Differential expression of uridine phosphorylase in tumors contributes to an improved fluoropyrimidine therapeutic activity. Mol. Cancer Ther. 10: 2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21: 70–71. [DOI] [PubMed] [Google Scholar]
- 18.Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. 1997. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407: 313–319. [DOI] [PubMed] [Google Scholar]
- 19.Cao D., Russell R. L., Zhang D., Leffert J. J., Pizzorno G. 2002. Uridine phosphorylase (-/-) murine embryonic stem cells clarify the key role of this enzyme in the regulation of the pyrimidine salvage pathway and in the activation of fluoropyrimidines. Cancer Res. 62: 2313–2317. [PubMed] [Google Scholar]
- 20.Pizzorno G., Wiegand R. A., Lentz S. K., Handschumacher R. E. 1992. Brequinar potentiates 5-fluorouracil antitumor activity in a murine model colon 38 tumor by tissue-specific modulation of uridine nucleotide pools. Cancer Res. 52: 1660–1665. [PubMed] [Google Scholar]
- 21.Samburova V., Lemos M. S., Hiibel S., Hoekman S. K., Cushman J. C., Zielinska B. 2013. Analysis of triacylglycerols and free fatty acids in algae using ultra-performance liquid chromatography mass spectrometry. J. Am. Oil Chem. Soc. 90: 53–64. [Google Scholar]
- 22.Seglen P. O. 1976. Preparation of isolated rat liver cells. Methods Cell Biol. 13: 29–83. [DOI] [PubMed] [Google Scholar]
- 23.Urasaki Y., Johlfs M. G., Fiscus R. R., Le T. T. 2012. Imaging immune and metabolic cells of visceral adipose tissues with multimodal nonlinear optical microscopy. PLoS ONE. 7: e38418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le T. T., Ziemba A., Urasaki Y., Brotman S., Pizzorno G. 2012. Label-free evaluation of hepatic microvesicular steatosis with multimodal coherent anti-Stokes Raman scattering microscopy. PLoS ONE. 7: e51092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans C. L., Xie X. S. 2008. Coherent anti-Stokes Raman scattering microscopy: chemically selective imaging for biology and medicine. Annu. Rev. Anal. Chem. 1: 883–909. [DOI] [PubMed] [Google Scholar]
- 26.Le T. T., Yue S., Cheng J. X. 2010. Shedding new light on lipid biology with coherent anti-Stokes Raman scattering microscopy. J. Lipid Res. 51: 3091–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldwin J., Farajallah A. M., Malmquist N. A., Rathod P. K., Phillips M. A. 2002. Malarial dihydroorotate dehydrogenase. Substrate and inhibitor specificity. J. Biol. Chem. 277: 41827–41834. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin J., Michnoff C. H., Malmquist N. A., White J., Roth M. G., Rathod P. K., Phillips M. A. 2005. High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. J. Biol. Chem. 280: 21847–21853. [DOI] [PubMed] [Google Scholar]
- 29.Moon A., Rhead W. J. 1987. Complementation analysis of fatty acid oxidation disorders. J. Clin. Invest. 79: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker U. A., Auclair M., Lebrecht D., Kornprobst M., Capeau J., Caron M. 2006. Uridine abrogates the adverse effects of antiretroviral pyrimidine analogues on adipose cell functions. Antivir. Ther. 11: 25–34. [PubMed] [Google Scholar]
- 31.Khutornenko A. A., Roudko V. V., Chernyak B. V., Vartapetian A. B., Chumakov P. M., Evstafieva A. G. 2010. Pyrimidine biosynthesis links mitochondrial respiration to the p53 pathway. Proc. Natl. Acad. Sci. USA. 107: 12828–12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizzorno G., Cao D., Leffert J. J., Russell R. L., Zhang D., Handschumacher R. E. 2002. Homeostatic control of uridine and the role of uridine phosphorylase: a biological and clinical update. Biochim. Biophys. Acta. 1587: 133–144. [DOI] [PubMed] [Google Scholar]
- 33.Knecht W., Henseling J., Loffler M. 2000. Kinetics of inhibition of human and rat dihydroorotate dehydrogenase by atovaquone, lawsone derivatives, brequinar sodium and polyporic acid. Chem. Biol. Interact. 124: 61–76. [DOI] [PubMed] [Google Scholar]
- 34.Lombard D. B., Alt F. W., Cheng H. L., Bunkenborg J., Streeper R. S., Mostoslavsky R., Kim J., Yancopoulos G., Valenzuela D., Murphy A., et al. 2007. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27: 8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirschey M. D., Shimazu T., Jing E., Grueter C. A., Collins A. M., Aouizerat B., Stancakova A., Goetzman E., Lam M. M., Schwer B., et al. 2011. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell. 44: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kendrick A. A., Choudhury M., Rahman S. M., McCurdy C. E., Friederich M., Van Hove J. L., Watson P. A., Birdsey N., Bao J., Gius D., et al. 2011. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem. J. 433: 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blander G., Guarente L. 2004. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 73: 417–435. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S., Xu W., Jiang W., Yu W., Lin Y., Zhang T., Yao J., Zhou L., Zeng Y., Li H., et al. 2010. Regulation of cellular metabolism by protein lysine acetylation. Science. 327: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S. C., Sprung R., Chen Y., Xu Y., Ball H., Pei J., Cheng T., Kho Y., Xiao H., Xiao L., et al. 2006. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell. 23: 607–618. [DOI] [PubMed] [Google Scholar]
- 40.Fritz K. S., Galligan J. J., Hirschey M. D., Verdin E., Petersen D. R. 2012. Mitochondrial acetylome analysis in a mouse model of alcohol-induced liver injury utilizing SIRT3 knockout mice. J. Proteome Res. 11: 1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creasey W. A., Hankin L., Handschumacher R. E. 1961. Fatty livers induced by orotic acid. I. Accumulation and metabolism of lipids. J. Biol. Chem. 236: 2064–2070. [PubMed] [Google Scholar]
- 42.Jung E. J., Kwon S. W., Jung B. H., Oh S. H., Lee B. H. 2011. Role of the AMPK/SREBP-1 pathway in the development of orotic acid-induced fatty liver. J. Lipid Res. 52: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becroft D. M., Phillips L. I., Simmonds A. 1969. Hereditary orotic aciduria: long-term therapy with uridine and a trial of uracil. J. Pediatr. 75: 885–891. [DOI] [PubMed] [Google Scholar]
- 44.Hirschey M. D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D. B., Grueter C. A., Harris C., Biddinger S., Ilkayeva O. R., et al. 2010. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 464: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwer B., Eckersdorff M., Li Y., Silva J. C., Fermin D., Kurtev M. V., Giallourakis C., Comb M. J., Alt F. W., Lombard D. B. 2009. Calorie restriction alters mitochondrial protein acetylation. Aging Cell. 8: 604–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong Y., Guan K. L. 2012. Mechanistic insights into the regulation of metabolic enzymes by acetylation. J. Cell Biol. 198: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Repa J. J., Inoue Y., Hayhurst G. P., Gonzalez F. J., Mangelsdorf D. J. 2004. Identification of a liver-specific uridine phosphorylase that is regulated by multiple lipid-sensing nuclear receptors. Mol. Endocrinol. 18: 851–862. [DOI] [PubMed] [Google Scholar]
- 48.Lavine J. E., Schwimmer J. B. 2004. Nonalcoholic fatty liver disease in the pediatric population. Clin. Liver Dis. 8: 549–558, viii–ix. [DOI] [PubMed] [Google Scholar]
- 49.Williams C. D., Stengel J., Asike M. I., Torres D. M., Shaw J., Contreras M., Landt C. L., Harrison S. A. 2011. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 140: 124–131. [DOI] [PubMed] [Google Scholar]
- 50.Petersen K. F., Befroy D., Dufour S., Dziura J., Ariyan C., Rothman D. L., DiPietro L., Cline G. W., Shulman G. I. 2003. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 300: 1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Begriche K., Massart J., Robin M. A., Borgne-Sanchez A., Fromenty B. 2011. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J. Hepatol. 54: 773–794. [DOI] [PubMed] [Google Scholar]
- 52.Cohen J. C., Horton J. D., Hobbs H. H. 2011. Human fatty liver disease: old questions and new insights. Science. 332: 1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angulo P. 2002. Treatment of nonalcoholic fatty liver disease. Ann. Hepatol. 1: 12–19. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.