Fig. 3.
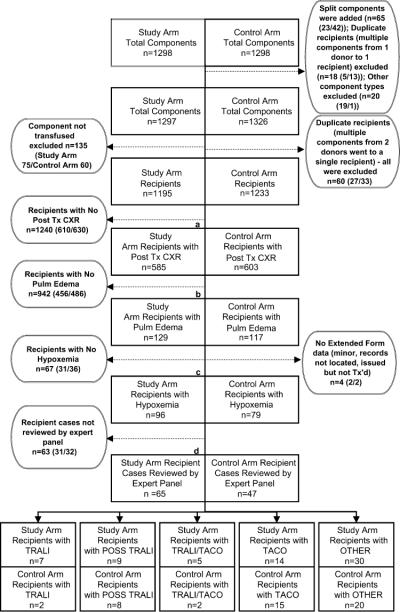
Results for LAPS-II component tracing and recipient medical record review in study and control arms. Boxes indicate number of recipients progressing through each stage of data collection. Circles indicate number of recipients completing data review at each stage and provide further details about recipients not progressing to the next stage of data collection. Due to space limitations, circles are displayed on either side of the primary flow chart. Within each circle, numbers in parentheses indicate study and control arm recipients. Split components are donations that were divided into two or more components that were transfused to different LAPS-II recipients, duplicate recipients are recipients who received more than one LAPS-II component and were excluded from the study for that reason, and other component types are components that were initially classified as high-plasma-volume components but which were volume reduced before transfusion. The percentage of study arm and control arm recipients who completed data collection at various junctures of the protocol was equivalent by exact p value of likelihood ratio statistic: (a) p = 0.98 for study or control arm by posttransfusion chest X-ray or no posttransfusion chest X-ray; (b) p = 0.26 for study or control arm by pulmonary edema or no pulmonary edema; (c) p = 0.23 for study or control arm by full or partial extended review; (d) p = 0.32 for study or control arm by expert panel review or no expert panel review. CXR = chest X-ray; Tx = transfusion.
