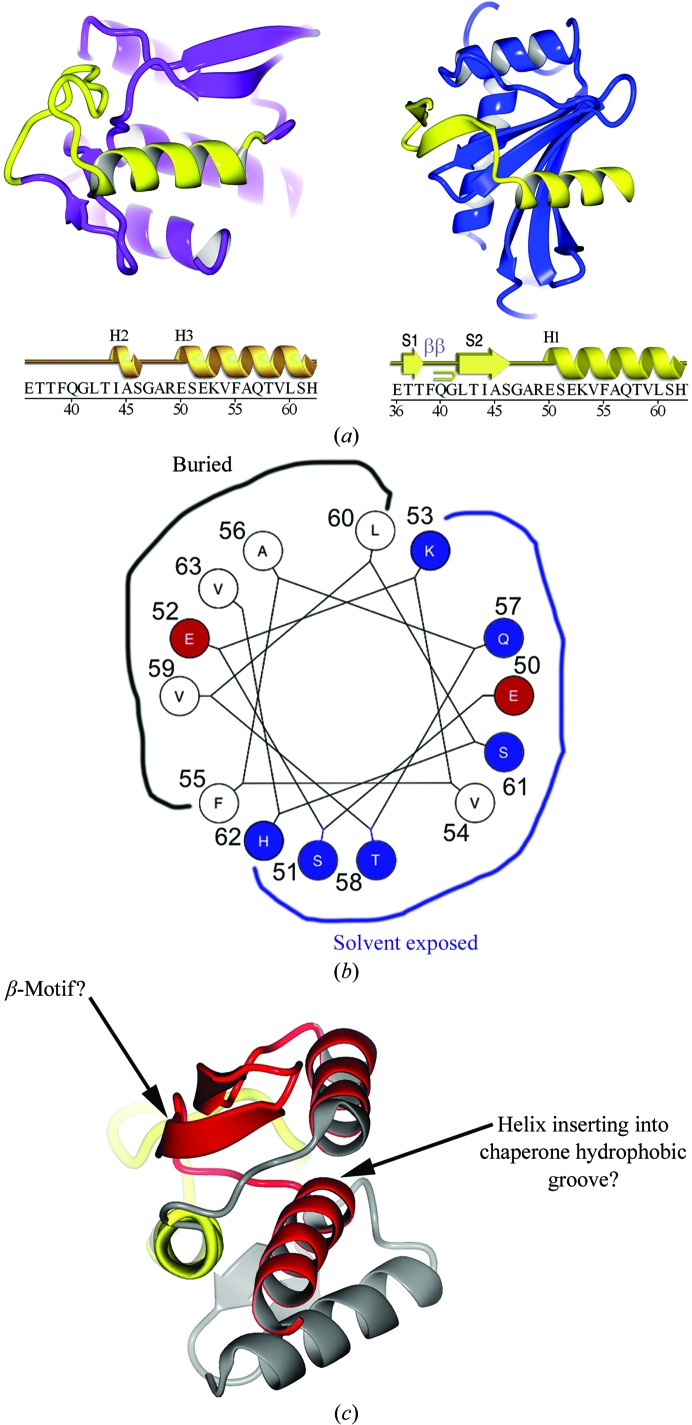
Figure 7.
Comparison of YopH peptides in the chaperone-bound and free states. (a) Ribbon diagram showing the YopH peptide 36–63 (yellow) in the context of the folded monomeric globular domain of YopH alone (left; YopH shown in purple outside of the 36–63 peptide) and in the context of the chaperone SycH (right; shown in blue). The sequence of the YopH peptide spanning residues 36–63 is shown below with secondary structure for each of the two structural contexts shown above them. Key: H, helices; S, strands; β, β-turn. (b) Helical wheel showing the interacting residues of YopH that bind in the SycH hydrophobic groove that are also used to form the hydrophobic core of the YopH globular fold. (c) The second SycH-binding peptide (shown in red) in YopH, with the hypothesized β-motif and amphipathic helix indicated.