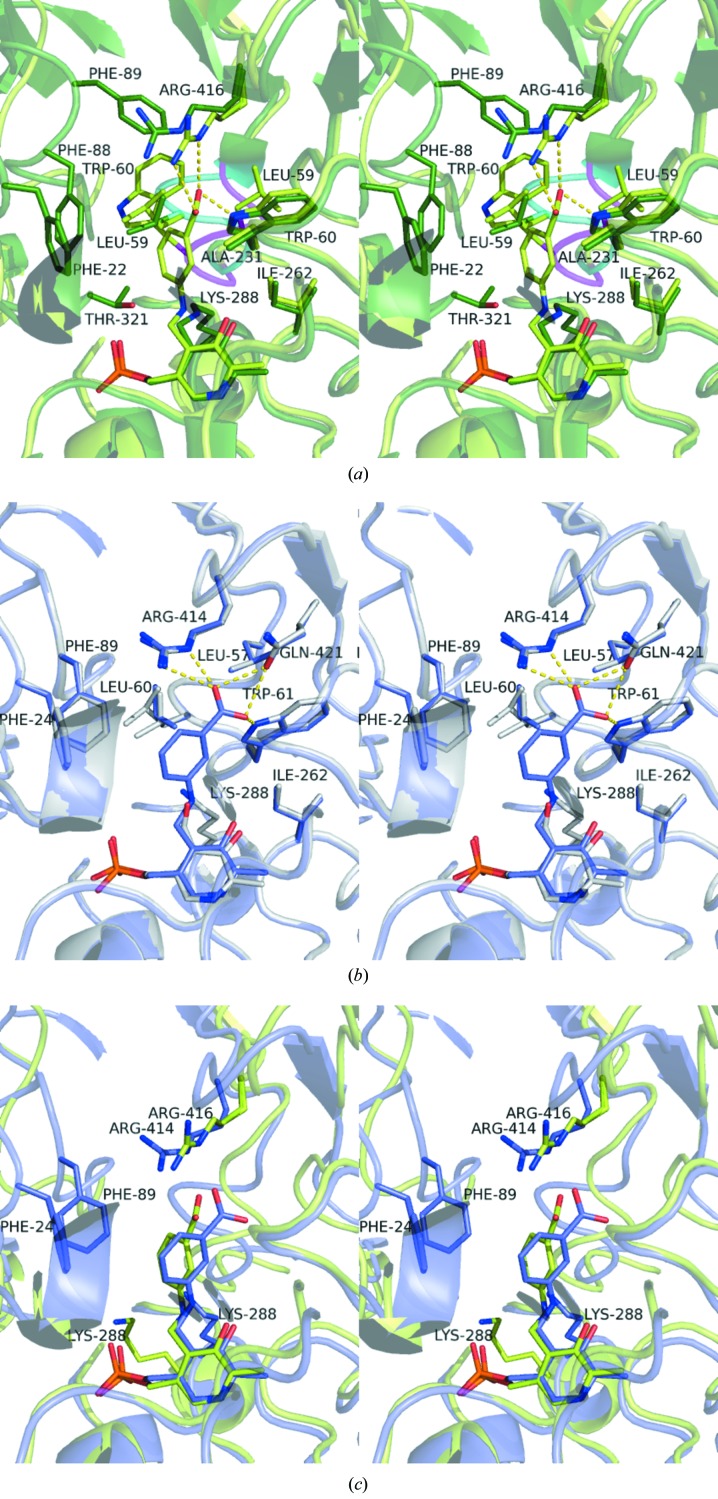
Figure 7.
Stereo representation comparing the gabaculine complexes of the C. violaceum Am:PyAT and P. aeruginosa β-A:PyAT enzymes. The side chains of residues within 4.5 Å of the mCPP inhibitor are shown as stick models. (a) The interactions of the mCPP bound in the C. violaceum Am:PyAT active site (light green). The residues of the holoenzyme are superimposed (dark green), highlighting the movements associated with inhibitor binding to the active site. The differences in the conformations of the Ala57–Cys61 loop are shown in magenta for the gabaculine-bound structure and in cyan for the holoenzyme structure. (b) The structure of mCPP-bound P. aeruginosa β-A:PyAT (blue) superimposed on the structure of its holoenzyme (grey). (c) The superposition of the active sites of the mCPP-complex structures of C. violaceum Am:PyAT (green) and P. aeruginosa β-A:PyAT (blue), highlighting the different orientations of mCPP observed between the two enzymes.