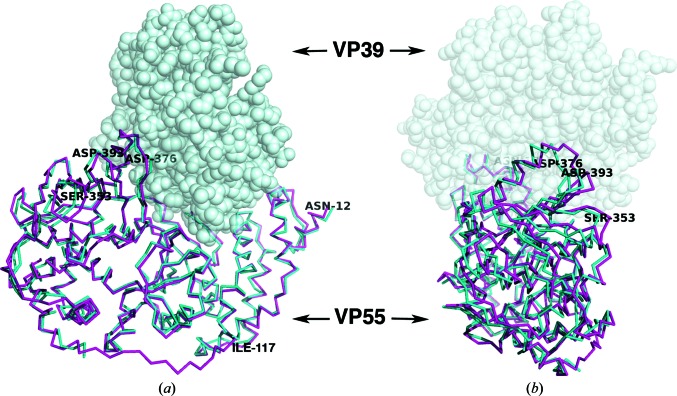
Figure 4.
Overlay of chain A in the VP55 monomer (magenta) and chain C in the VP39–VP55 heterodimer (PDB entry 3erc; cyan) based on a superposition of the N-terminal domain (residues 12–117) only. VP39, as it would be docked in the heterodimer, is shown as a gray space-filling model. In (a) the N-terminal domain is on the right while the C-terminal domain is on the left. (b) is a view rotated 90° with respect to (a) such that the C-terminal domain of VP55 is facing the viewer. The residues discussed in the text are labeled. The ‘rocking’ motion of the C-terminal domain of VP55 is exemplified by the large r.m.s.d. in Cα positions of Ser353 and Asp393 (Table 2 ▶). Although molecules A and B are both shown in the context of VP39 to highlight changes to the VP39-binding site, we have no evidence that VP39 can bind VP55 in the conformation of molecule A; in fact, we suggest that it does not.