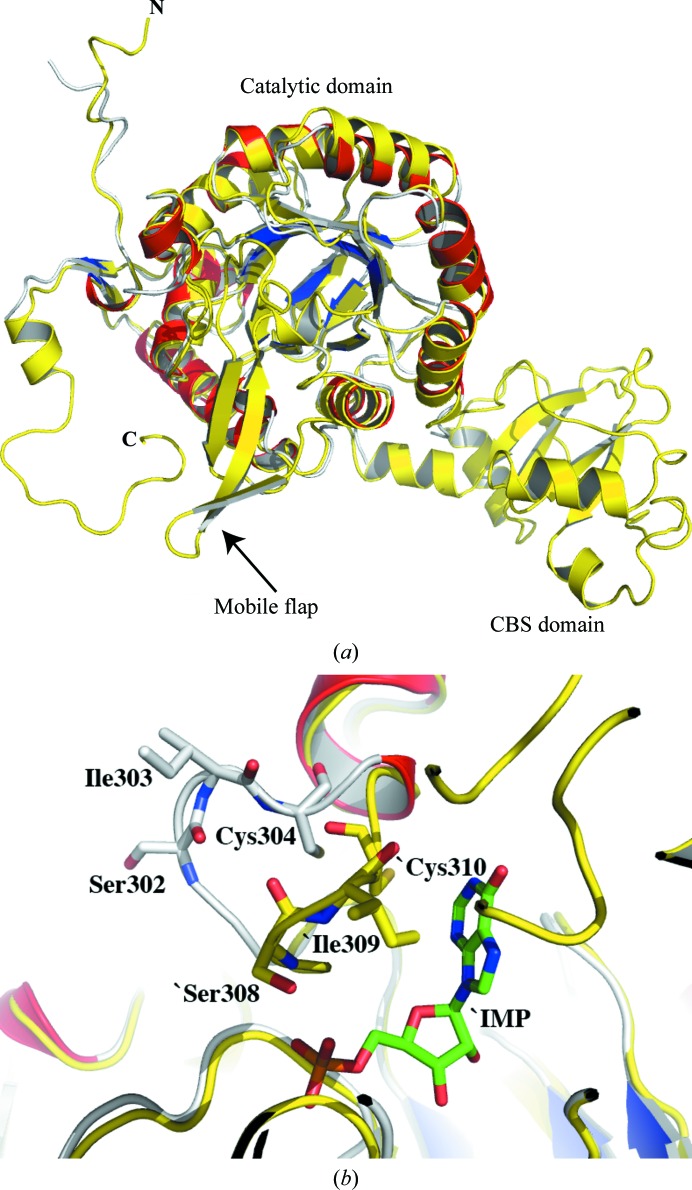
Figure 2.
(a) Superposition of PaIMPDH (grey main-chain trace with red helices and blue strands) and S. pyogenes IMPDH (all yellow; PDB entry 1zfj) matches 293 residues with an r.m.s.d. of 1.1 Å based on a least-squares fit of Cα positions and highlights the missing residues in PaIMPDH as corresponding to the CBS subdomain, a mobile flap and C-terminal regions. (b) Close-up of the active site. The active-site loop of PaIMPDH (grey) is fully ordered and adopts an ‘open’ conformation compared with the ‘closed’ conformation observed when a ligand is bound to the S. pyogenes enzyme (yellow). The distances from the catalytic cysteine Sγ atoms to IMP C2 are approximately 6 and 3 Å in PaIMPDH and SpIMPDH, respectively.