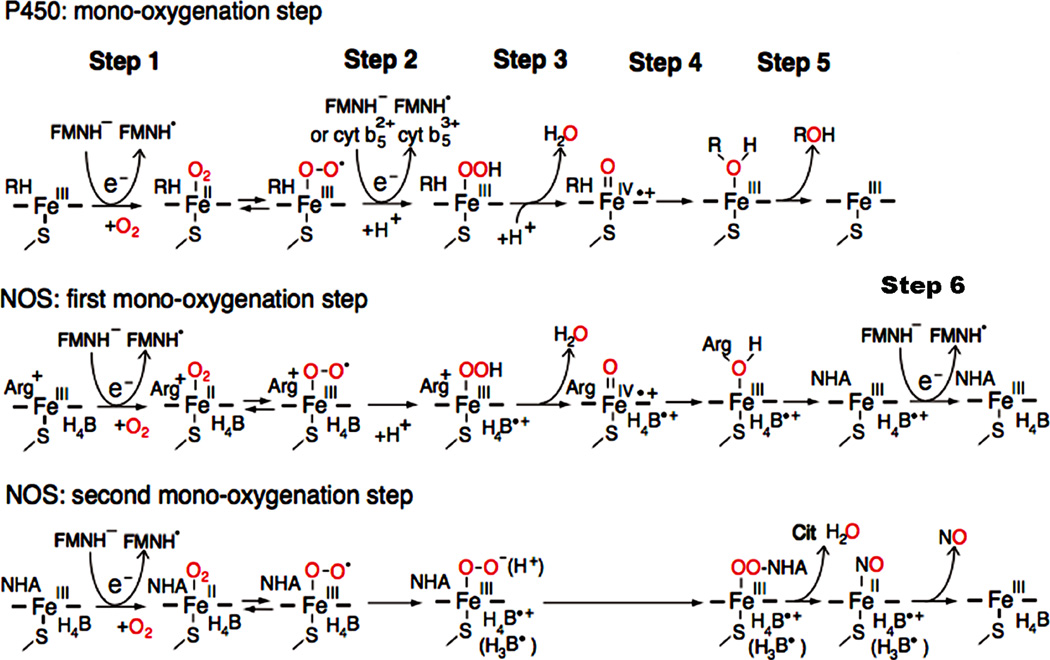
Figure 11.
The role of the FAD-FMN pair for activation of molecular oxygen in P450 and the NOS heme center. In P450, introduction of the first electron involves the reduction of ferric P450 (FeIII) and binding O2 (step 1). The addition of the second electron leads to the production of the ferric peroxy form, which is rapidly protonated to the ferric hydroperoxy state (step 2). A further protonation facilitates the ionic O-O bond clevage (step 3), and resulting in a ferryl-oxo form, Compound I, FeIV=O + species [152, 210, 211]. In final steps, 4 and 5, the “oxygen” of the Compound I is transferred to substrate (RH). In NOS, the first electron is also supplied by FMNH−, but the second electron is supplied by the bound H4B (see text for details). H4B functions as a one-electron carrier: H4B ⇔ H4B.+ + e−, and can also function as an electron and proton donor: H4B ⇔ H3B. + e− + H+ (shown in parentheses) [151, 212, 213]. NOS also requires step 6. Figure adapted from [214].