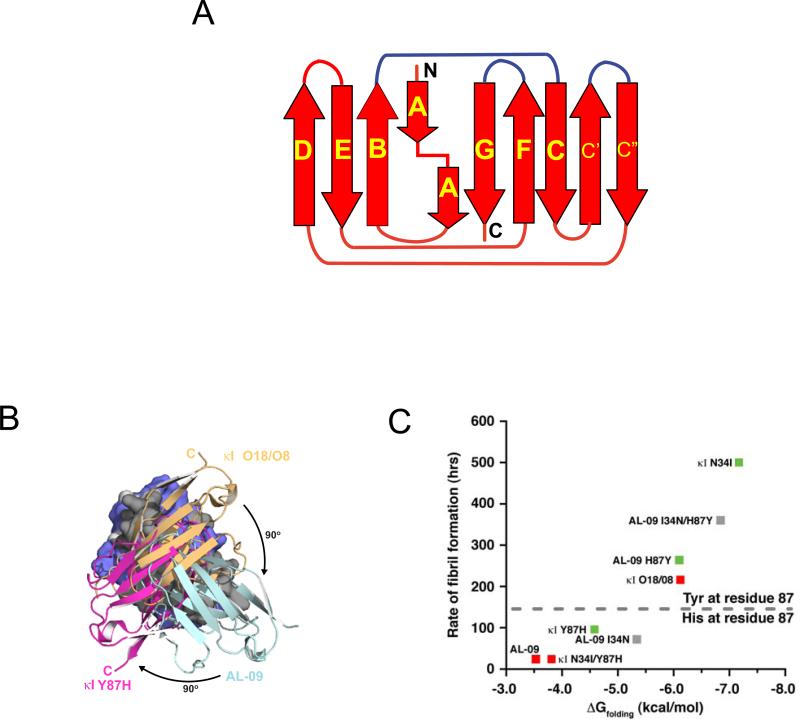
Figure 1.
A) Schematic representation of the light chain variable domain. The b-strands are labeled as arrows and with their respective letter names. The regions corresponding to the complementarity determining regions (CDRs) are colored in blue. B) Overlay of three different dimer orientations found in crystal structures of κI O18/O8, AL-09, and NMR structure of κI Y87H. C) Rates of fibril formation are inversely correlated with ΔGfolding. Mutation of Y87 to Histidine stabilizes an altered dimer interface and promotes faster rates of amyloid formation. HSQC analysis was used to categorize each protein as single state (green) or promiscuous states (red); others were not determined (gray). Figures B and C adapted from (Peterson et al., 2010, Fig. 4 and 5, respectively), with permission from Elsevier. Copyright © 2010.