Abstract
Aim: The aim of the present study was to explore the role of receptor tyrosine kinases (RTKs) in the regulation of expression of PTX-3, a protector in atherosclerosis. Methods: Human monocytic U937 cells were infected with a shRNA lentiviral vector library targeting human RTKs upon LPS stimuli and PTX-3 expression was determined by ELISA analysis. The involvement of downstream signaling in the regulation of PTX-3 expression was analyzed by both Western blotting and ELISA assay. Results: We found that knocking down of ERBB2/3, EPHA7, FGFR3 and RET impaired PTX-3 expression without effects on cell growth or viability. Moreover, inhibition of AKT, the downstream effector of ERBB2/3, also reduced PTX-3 expression. Furthermore, we showed that FGFR3 inhibition by anti-cancer drugs attenuated p38 activity, in turn induced a reduction of PTX-3 expression. Conclusion: Altogether, our study demonstrates the role of RTKs in the regulation of PTX-3 expression and uncovers a potential cardiotoxicity effect of RTK inhibitor treatments in cancer patients who have symptoms of atherosclerosis or are at the risk of atherosclerosis.
Keywords: PTX-3, RNAi screening, RTKs, target therapy, cardiotoxicity, atherosclerosis
Introduction
Treatment of patients with cancer has changed radically over the last several years with the advent of “targeted therapeutics.” This targeted approach, predominantly via inhibition of tyrosine kinase activity, has markedly improved the management of cancers. Given the initial success of this approach, the number of targeted therapy drugs entering into development in the last 5 years has increased dramatically. And so far, there are hundreds of kinase inhibitors somewhere in between discovery and market, with 80% of drug development being in cancer [1].
Although target therapy has an improve antitumor activity with fewer toxic side effects than traditional anticancer therapies including radiation therapies and chemotherapies, many approved RTK inhibitors have been found to evoke cardiac dysfunction in some cancer patients [2-4] . Furthermore, there is a strong link between kinase pathway inhibition and cardiotoxicity as compared with other organ toxicities [5-7]. However, in many cases, adverse cardiac events in the clinic were not anticipated based on the fact that predefined cardiac endpoints was not included in early clinical trials and the difficulties to diagnose heart failure in patients with cancer. Since kinase inhibition has revolutionized the treatment of cancer, which can be managed effectively for years and could be eventually regarded as a chronic disease, one could except that the rate of cardiotoxicity in cancer patients treated with kinase inhibitors would increase in future.
Dysfunctions of cardiovascular system induced by kinase inhibitor therapy are varied and have included heart failure, LV dysfunction, conduction abnormalities, QT prolongation, acute coronary syndromes, myocardial injury, arterial thromboses and hypertension [8]. Since atherosclerosis is considered as the significant underlying cause of cardiovascular disease (CVD) [9,10], inhibition of driver kinases might therefore potentially compromise the cardiovascular system function by accelerating atherosclerosis. However, the side effects of treatment with kinase inhibitors on atherosclerosis in cancer patients have not been fully investigated due to lack of appropriate preclinical model.
Given the critical role of inflammation in atherosclerosis, circulating factors related to inflammation and atherosclerosis therefore attracted attention [11]. Of interest, pentraxin protein family is highly associated with CVD [11]. Pentraxin protein family consists of C-reactive protein (CRP), serum amyloid P component (SAP) and pentraxin-3 (PTX-3) [11-13], all of which have the features of pattern recognition receptors and are involved in human humoral immune response [14,15]. In contrast to CRP, PTX-3 demonstrates to be more specifically associated with advanced atherosclerosis [15-17]. PTX-3 is highly expressed in advanced atherosclerosis tissues [18,19], including macrophages, surviving endothelial cells, activated monocytes and infiltrating neutrophils [19]. Amounts of evidence suggest the possibility that the increased levels of PTX-3 in subjects with CVD may reflect a protective physiologic response that correlates with the severity of the disease [20-22]. More importantly, deficiency of the long pentraxin PTX-3 promotes vascular inflammation and atherosclerosis [23].
To systematically investigate the function of receptor tyrosine kinases (RTKs), the main targets in target cancer therapies, on atherosclerosis, we set out to screen a short hairpin RNA (shRNA) library representing the full complement of 56 human RTKs (Table 1) for genes whose inhibition could impair PTX-3 expression in U937 cells. Our results revealed multiple functionally important pathways, particularly the FGFR/p38 signaling in the regulation of PTX-3 expression. Our study suggests that clinically used kinase inhibitor might contribute to atherosclerosis, in turn inducing cardiotoxicity, by interfering with PTX-3 expression. These findings could provide a preclinical model for studying the side effects of kinase inhibitors on atherosclerosis.
Table 1.
RTKs genes for screen
| Gene symbol | Official Full Name | Inhibitor | Associated with cardiotoxicity (Ref) |
|---|---|---|---|
| ERBB4 | v-erb-a erythroblastic leukemia viral oncogene homolog 4 | NA | NA |
| MST1R | macrophage stimulating 1 receptor | NA | NA |
| RYK | receptor-like tyrosine kinase | NA | NA |
| KDR | kinase insert domain receptor | sorafenib/sunitinib | [8,32] |
| NTRK1 | neurotrophic tyrosine kinase, receptor, type 1 | NA | NA |
| STYK1 | serine/threonine/tyrosine kinase 1 | NA | NA |
| EPHA3 | EPH receptor A3 | NA | NA |
| FLT1 | fms-related tyrosine kinase 1 | sorafenib/sunitinib | [8,32] |
| FGFR1 | fibroblast growth factor receptor 1 | PD-173074 | NA |
| AXL | AXL receptor tyrosine kinase | NA | NA |
| NTRK2 | neurotrophic tyrosine kinase, receptor, type 2 | NA | NA |
| TEK | TEK tyrosine kinase, endothelial | NA | NA |
| EGFR | epidermal growth factor receptor | erlotinib/gefitinib/lapatinib | [1,8] |
| ROR2 | receptor tyrosine kinase-like orphan receptor 2 | NA | NA |
| FLT3 | fms-related tyrosine kinase 3 | sorafenib/sunitinib | [8,32] |
| ALK | anaplastic lymphoma receptor tyrosine kinase | crizotinib | NA |
| ERBB3 | v-erb-b2 erythroblastic leukemia viral oncogene homolog 3 (avian) | AZD8931 | NA |
| ERBB2 | v-erb-b2 erythroblastic leukemia viral oncogene homolog 2 | Lapatinib | [1,32] |
| INSRR | insulin receptor-related receptor | NA | NA |
| EPHA6 | EPH receptor A6 | NA | NA |
| EPHA5 | EPH receptor A5 | NA | NA |
| EPHA7 | EPH receptor A7 | NA | NA |
| FGFR3 | fibroblast growth factor receptor 3 | Linifanib/AZD4547 | NA |
| MUSK | muscle, skeletal, receptor tyrosine kinase | NA | NA |
| RET | ret proto-oncogene | Motesanib | [8,32] |
| IGF1R | insulin-like growth factor 1 receptor | NVP-ADW742 | NA |
| CSF1R | colony stimulating factor 1 receptor | Linifanib | [8] |
| MET | met proto-oncogene (hepatocyte growth factor receptor) | AMG-208 | NA |
| EPHA2 | EPH receptor A2 | NA | NA |
| PDGFRB | platelet-derived growth factor receptor, beta polypeptide | Dasatinib/nilotinib | [1,8,32] |
| INSR | insulin receptor | NA | NA |
| FGFR2 | fibroblast growth factor receptor 2 | AZD4547 | NA |
| PTK7 | PTK7 protein tyrosine kinase 7 | NA | NA |
| KIT | v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | nilotinib/sorafenib | [1,8,32] |
| PDGFRA | platelet-derived growth factor receptor, alpha polypeptide | Dasatinib/sunitinib | [1,8,32] |
| TIE1 | tyrosine kinase with immunoglobulin-like and EGF-like domains 1 | NA | NA |
| DDR2 | discoidin domain receptor tyrosine kinase 2 | NA | NA |
| DDR1 | discoidin domain receptor tyrosine kinase 1 | NA | NA |
| LTK | leukocyte receptor tyrosine kinase | NA | NA |
| EPHB3 | EPH receptor B3 | NA | NA |
| FGFR4 | fibroblast growth factor receptor 4 | BGJ398 | NA |
| EPHA8 | EPH receptor A8 | NA | NA |
| NTRK3 | neurotrophic tyrosine kinase, receptor, type 3 | NA | NA |
| FLT4 | fms-related tyrosine kinase 4 | sunitinib | [8,32] |
| EPHB4 | EPH receptor B4 | NA | NA |
| LMTK2 | lemur tyrosine kinase 2 | NA | NA |
| EPHA1 | EPH receptor A1 | NA | NA |
| AATK | apoptosis-associated tyrosine kinase | NA | NA |
| MERTK | c-mer proto-oncogene tyrosine kinase | NA | NA |
| TYRO3 | TYRO3 protein tyrosine kinase | BMS 777607 | NA |
| ROS1 | c-ros oncogene 1, receptor tyrosine kinase | NA | NA |
| EPHA4 | EPH receptor A4 | NA | NA |
| EPHB6 | EPH receptor B6 | NA | NA |
Ref, reference; NA, not available.
Materials and methods
Cell culture
293T cells (ATCC) were cultured in DMEM medium supplemented with 10% FBS (Hyclone). U937 cells (ATCC, CRL-1593.2) were grown in RPMI 1640 medium supplemented with 10% FBS (Hyclone). All media were supplemented with penicillin (100 IU·ml-1) and Streptomycin (100 μg·ml-1) (Life Technologies).
Induction of PTX-3 expression by LPS stimuli
The cells were washed and incubated in endotoxin-free RPMI 1640 (10% FBS) at 5 X 104 cells each well in 24-well plate, with or without LPS (lipopolysaccharide, Escherichia coli 0127:B8, Sigma, L4516 ) stimuli for 6 hrs at 37°C in the presence of 5% CO2. The optimized concentration of LPS to induce PTX-3 expression was tested with 0, 20, 50 and 100 ng·ml-1.
U937 cells induced by series of concentration of LPS were homogenized with 0.5 ml Trizol (life technologies) and total RNA was isolated as instruction manual (life technologies). Reverse transcription was carried out at 42°C for 1 hour with oligo dT. Real-time PCR primers were designed as follows: forward primer: tgtatgtgaatttggacaacgaa; reverse primer: cattccgagtgctcctgac. Reaction program was 2 min at 95°C, 40 cycles of 95°C for 30 s, 60°C for 30 s and 72°C for 30 s. The concentration of LPS induced the maximal expression of PTX-3 was selected to carry out RNAi screening.
RNAi screening
U937 cells were incubated in endotoxin-free RPMI 1640 (10% FBS) at 1 × 104 cells each well in 96-well plates, and then infected respectively with 55 RTK lentiviral shRNAs in the presence of polybrene to the final concentration of 8 μg·ml-1, each gene in the library was duplicate in two plates. LPS was added to cell culture to final concentration 100 ng·ml-1 120 h after infection for 6 hrs. After the treatment, cells were pelleted and divided into two parts: the supernate was collected to measure PTX-3 levels by ELISA, and the pellets were used to detect cell viability by MTS assay (Promega, G3580) according to the operation manual.
Determination of PTX-3 expression by ELISA assay
PTX-3 levels were measured by the Sandwich ELISA as previously described. Briefly, ELISA plates were coated with 100 ng each well of mouse anti-human PTX-3 antibody diluted in PBS by overnight incubation at 4°C. Washing buffer (PBS containing 0.05% Tween 20) was used to wash plates thoroughly after each step. Non-specific binding to the plates was blocked with 2% BSA in PBS for 2 h at room temperature before adding unknown samples. After incubation for 2 h at room temperature, 10 ng each well of biotin conjugated anti-PTX-3 goat IgG were then added (1 h at room temperature) followed by the addition of 50 μl of streptavidin-peroxidase. Finally, 100 μl of Substrate Solution (1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine), R&D Systems Catalog # DY999) were added and absorbance values were read at 450 nm in an automatic ELISA reader.
Protein isolation and western blotting
Cell pellets were resuspended in 1×SDS loading buffer (1 mmol·L-1 Na3VO4, 10 mmol·L-1 NaF, 1 mmol·L-1 PMSF) containing protease inhibitors. Lysates (20 μg each lane) were applied to SDS-PAGE. Immunoblotting of Abs specific for GAPDH (Abmart, 080922), p38 (A-12, Santa Cruz, sc-7972) and p-p38 (D-8, Santa Cruz, SC-7973) were detected using HRP-conjugated anti-mouse (Promega) and visualized by chemiluminescence detection system (Millipore, WBKLS0500).
Results
Loss-of-function screen for RTKs regulating PTX-3 expression
PTX-3 is produced by macrophages and a variety of tissue cells upon exposure to LPS, which has been commonly used as a reagent to induce inflammatory response [15]. Consistent with previous reports, we found a dose-dependent induction in PTX-3 expression after LPS stimulation in monoblastic U937 cells (Figure 1A). We therefore analyze PTX-3 expression in U937 cells after treatment with LPS at 100 ng·ml-1 for 6 hrs with or without other treatment in our experiments.
Figure 1.
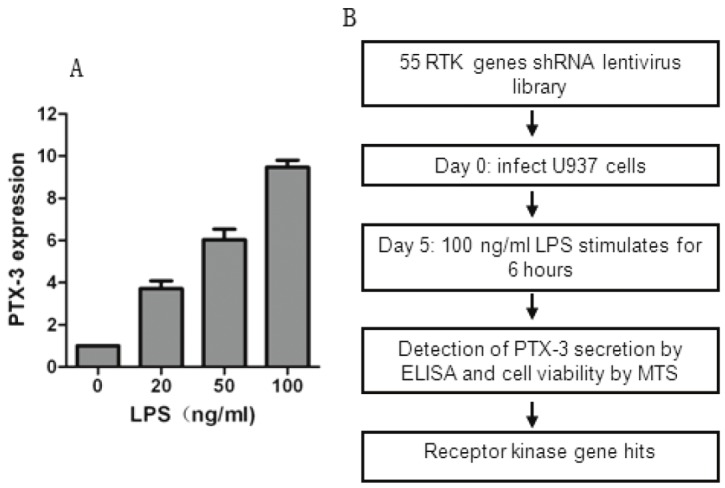
Schematic of RNAi screen. A. U937 cells were treated with 100 ng·ml-1 of LPS for 6 hrs and PTX-3 expression was determined by qPCR analysis. B. Schematic outline of high-throughput RNAi screen. The human RTKs lentiviral vector library was used to infect U937 cells for 4 days to allow efficient knocking down of RTKs and was then treated with LPS at 100 ng·ml-1 for 6 hrs. The supernatant media and U937 cells were collected by centrifuged and analyzed by ELSIA and MTS assay, respectively. In total, all human 55 RTKs were analyzed.
Given that RTKs have been widely demonstrated as most important driver genes for tumor development, many small molecular inhibitors are designed to inhibit RTKs and a bunch of RTK inhibitors have been extensively used in both cancer treatment and clinical trials. Hence, the lost-of-function screen used a shRNA library targeting human RTKs involved in cancer, in which each shRNA induces strong and specific suppression of gene expression over prolonged period of time. We configured the screen to allow the identification of RTKs that regulate the expression of PTX-3 in U937 cells (Figure 1B). The output of the screen is described in Table S1 available online. The screen was carried out in duplicate and the expression of PTX-3 was detected by ELISA as described in Material and Methods. Genes that could affect survival and proliferation of U937 cells were also obtained by MTS assay (Table S1). The ratio of the ELISA to MTS value after infection with RTKs shRNA lentiviral libraries and the mean value of the reduction of ELISA/MTS was calculated (Table S1 and Figure 2A). We set a cutoff for gene with > 30% of reduction in the value of ELISA/MTS to be considered potential candidates, and 5 genes were obtained (Figure 2B).
Figure 2.
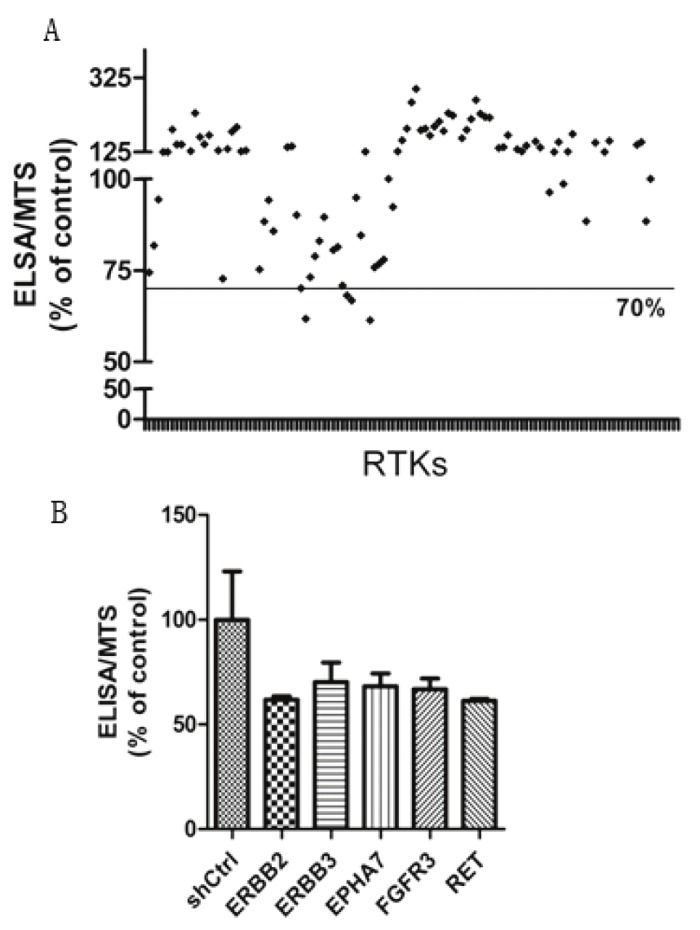
RNAi screen for RTKs required for PTX-3 secretion induced by LPS. A. Scatter plot of PTX-3 relative secretion levels for each RTK gene in U937 cells infected by two independent shRNAs per gene. The relative secretion levels of PTX-3 were represented by ratio of the ELISA absorbance to the MTS absorbance. B. Candidate genes with an inhibition rate of >30% were listed.
Characterization of RTKs with respect to PTX-3 expression
Several of the positive regulators of PTX-3 expression we identified were already shown to have cardiotoxicity. Of particular interest were ERBB2 and ERBB3, inhibitors of which are widely used in breast cancer target therapy [24]. Expression of both ERBB2 and ERBB3 is usually enhanced during tumorigenesis, resulting in promotion of cell proliferation and inhibition of apoptosis [25]. We found a 30-40% of reduction in PTX-3 expression after either ERBB2 or ERBB3 inhibition (Table 1), indicating the potential toxicity of ERBB2/3 signaling inhibition by influencing atherosclerosis regulation. Additionally, we identified RET as a regulator of PTX-3 expression (Figure 2B). Knocking down of RET expression induced a 40% of reduction in PTX-3 expression (Table 1). Interestingly, RET inhibitor, sunitinib, has also been widely recognized as an inducer of cardiotoxicity [3,26].
Since AKT is a canonical downstream signaling of ERBB2/3 signaling and AKT inhibitor is a promising anti-cancer drug on clinical trials, we examine whether inhibition of AKT signaling could also impair PTX-3 expression. We treated U937 cells with AKT inhibitor, MK2206, which is on clinical trials and found that, similar with inhibition of ERBB2/3 signaling, AKT signaling inhibition reduced PTX-3 expression (Figure 3), suggesting that ERBB2/3 might regulate PTX-3 expression via AKT signaling and could in turn influence atherosclerosis.
Figure 3.

The downstream signaling involved in RTKs regulating PTX-3 secretion induced by LPS. U937 cells were pretreated with AKT inhibitor MK2206 for 24 hrs and then added with LPS for another 6 hrs. U937 cells were centrifuged and analyzed by MTS assay. The supernatant were collected and PTX-3 secretion was determined by ELISA assay. The ELISA/MTS value was calculated as described in Materials and methods.
Identification of p38 signaling in the regulation of atherosclerosis dysfunction
FGFR3 has been shown to drive oncogenesis in a subset of patients with multiple myeloma and some epithelial cancers. We found that knocking down of FGFR3 expression induced a 30% of reduction in PTX-3 expression (Figure 2B). Notably, FGFR3 inhibition had no effect on cell viability (Figure S1), indicating that the inhibition of PTX-3 expression is not due to cytotoxicity.
Next, we explored the mechanism by which FGFR3 regulates PTX-3 expression. Since p38 signaling has an important role in the regulation of atherosclerosis [27], we examined p38 signaling activity after FGFR3 inhibition. We found that treatment of Linifanib, a potent FGFR3 inhibitor, induced a dose-dependent reduction of phosphorylation of p38 (Figure 4B, C). Consistently, p38 inhibitor treatment impaired LPS-induced PTX-3 expression (Figure 4A). Collectively, these data demonstrated a link between FGFR3-p38 signaling and the regulation of PTX-3 expression.
Figure 4.

FGFR3-p38 signaling regulates PTX expression. A. p38 inhibitor LY2228820 reduced the PTX-3 secretion induced by LPS in U937 cells.( **, p<0.01; ***, p<0.001), Error bars show data ±standard error. Means were derived from three replicates. B. Western blot analysis of p38 phosphorylation in U937 cells treated by RTK inhibitor Linifanib. U937 cells were treated with FGFR3 inhibitor, Linifanib, with different doses for 24 hrs and centrifuged for western blot analysis. Whole cell lysates were loaded for the expression analysis of phosphorylation of p38 and total p38. C. Optical density analysis of western blot in B. PTX-3 secretion and U937 cell viability was detected by ELISA and MTS assay, respectively. The ELISA/MTS value was calculated as described in Materials and methods.
Discussion
Cardiovascular disease, a leading cause of mortality in developed countries, is mainly caused by atherosclerosis, a chronic inflammatory disease. Atherosclerosis is referred to as a hardening or furring of the arteries, in which an artery wall thickens as a result of the accumulation of fatty materials such as cholesterol. PTX-3, a molecule acting as the humor arm of innate immunity, is produced by the major cell types in atherosclerotic lesion in response to inflammatory stimuli. Previous reports points out a potential protective effect of PTX-3 in the atherosclerotic, which PTX-3 deficiency is associated with increased atherosclerosis in apolipoprotein-E-deficient mice and increased macrophage accumulation in the atherosclerotic lesions.
Although growing evidences show a cardiotoxicity induced by target therapies with kinase inhibitors, little is known about the side effects on atherosclerosis. In this study, we demonstrate the utility of an RNAi-based screen to identify molecules required for the regulation of PTX-3 expression, which is critical for the protection of atherosclerosis. The results presented here raise the possibility that treatment with multiple RTK inhibitors contributes to the accelerating process of atherosclerosis because the expression of PTX-3, an important protector of atherosclerosis, is impaired. To our knowledge, it is the first time to systematically explore the side effect of RTK inhibition on atherosclerosis.
Previous report which studied FGFR3 expression between normal and athermanous human arteries show that FGFR3 exhibited more restricted patterns of distribution within the plaque [28], suggesting an important role of FGFR3 in the regulation of atherogenesis. Consistent with this, we demonstrate that depleting FGFR3 expression induced a reduction of PTX-3 expression via p38 signaling. While FGFR3 functions in tumor development and therefore is a promising drug target for cancer therapy [29,30], our evidence suggests a possibility of cardiotoxicity side effect when treating cancer patients with FGFR3 inhibitor. Further analysis about the markers associated with atherosclerosis in either animal models or target therapy treated cancer patients who have less severe atherosclerosis are needed to demonstrate in vivo effects.
Moreover, we provide a novel explanation about the cardiotoxicity effect of cancer therapies targeting ERBB2/3. Retrospective studies have reported incidences of symptoms of heart failure or LV dysfunction as high as 35% causing discontinuation of therapy in 20% of cancer patients with ERBB2/3 inhibition treatment [31]. Here, we found that ERBB2/3 inhibition impairs PTX-3 expression, indicating a highly possibility that ERBB2/3 inhibition therapy might accelerate the risk of atherosclerosis and in turn induce cardiotoxicity. Importantly, we also explored the potential mechanism by which ERBB2/3 inhibition impairs PTX-3 expression and found the involvement of AKT signaling in this side effect. It is worth noting that our results suggest the therapy involving an AKT inhibitor need to be careful when cancer patients either have symptoms of atherosclerosis or are at high risk of atherosclerosis.
In conclusion, we have used a RTKs-based loss-of-function screening approach to systematically explore the link between RTKs, common cancer drug targets, and atherosclerosis, one of the most common cardiovascular diseases. Our study has unearthed a novel mechanism by which multiple RTK inhibition-based cancer therapies could induce cardiotoxicity via accelerating atherosclerosis.
Acknowledgments
We gratefully acknowledge Lei Xiong, Feng-Qing Li, Lin Shi and Li Zhang for helpful discussion, Tao Zhou for the technical supports of western blot analysis. This work was supported by grants of The National Natural Science Foundation of China (81000087), Basic Research of Science and Technology Commission of Shanghai Municipality (10JC1414000), Scientific Research Project of City Health Bureau of Shanghai (20114Y092) and The Development of Science and Technology Fund Project of Shanghai Chest Hospital (YZ11-07).
Supporting Information
References
- 1.Force T, Kerkela R. Cardiotoxicity of the new cancer therapeutics--mechanisms of, and approaches to, the problem. Drug discovery today. 2008;13:778–784. doi: 10.1016/j.drudis.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 3.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM TARGET Study Group. Sorafenib in advanced clear-cell renalcell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. 2010;106:35–46. doi: 10.1161/CIRCRESAHA.109.205906. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Zuppinger C, Suter TM. Cancer therapy-associated cardiotoxicity and signaling in the myocardium. J Cardiovasc Pharmacol. 2010;56:141–146. doi: 10.1097/FJC.0b013e3181e0f89a. [DOI] [PubMed] [Google Scholar]
- 8.Chen MH, Kerkela R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118:84–95. doi: 10.1161/CIRCULATIONAHA.108.776831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 11.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 12.Breviario F, d’Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 13.Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993;150:1804–1812. [PubMed] [Google Scholar]
- 14.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28:1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 16.Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493. [PubMed] [Google Scholar]
- 17.Luchetti MM, Piccinini G, Mantovani A, Peri G, Matteucci C, Pomponio G, Fratini M, Fraticelli P, Sambo P, Di Loreto C, Doni A, Introna M, Gabrielli A. Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA) Clin Exp Immunol. 2000;119:196–202. doi: 10.1046/j.1365-2249.2000.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–4. doi: 10.1161/01.atv.0000015595.95497.2f. [DOI] [PubMed] [Google Scholar]
- 19.Savchenko A, Imamura M, Ohashi R, Jiang S, Kawasaki T, Hasegawa G, Emura I, Iwanari H, Sagara M, Tanaka T, Hamakubo T, Kodama T, Naito M. Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol. 2008;215:48–55. doi: 10.1002/path.2314. [DOI] [PubMed] [Google Scholar]
- 20.Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med. 2010;20:35–40. doi: 10.1016/j.tcm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Garlanda C, Bottazzi B, Moalli F, Deban L, Molla F, Latini R, Mantovani A. Pentraxins and atherosclerosis: the role of PTX3. Curr Pharm Des. 2011;17:38–46. doi: 10.2174/138161211795049750. [DOI] [PubMed] [Google Scholar]
- 22.Kotooka N, Inoue T, Fujimatsu D, Morooka T, Hashimoto S, Hikichi Y, Uchida T, Sugiyama A, Node K. Pentraxin3 is a novel marker for stent-induced inflammation and neointimal thickening. Atherosclerosis. 2008;197:368–374. doi: 10.1016/j.atherosclerosis.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F, Pizzitola I, Garlanda C, Mantovani A, Catapano AL. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez RH, Valero V, Hortobagyi GN. Emerging targeted therapies for breast cancer. J. Clin. Oncol. 2010;28:3366–3379. doi: 10.1200/JCO.2009.25.4011. [DOI] [PubMed] [Google Scholar]
- 25.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, Sánchez V, Koland J, Muller WJ, Arteaga CL, Cook RS. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res. 2012;72:2672–2682. doi: 10.1158/0008-5472.CAN-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19:1613–1618. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 27.Seeger FH, Sedding D, Langheinrich AC, Haendeler J, Zeiher AM, Dimmeler S. Inhibition of the p38 MAP kinase in vivo improves number and functional activity of vasculogenic cells and reduces atherosclerotic disease progression. Basic Res Cardiol. 2010;105:389–397. doi: 10.1007/s00395-009-0072-9. [DOI] [PubMed] [Google Scholar]
- 28.Hughes SE. Localisation and differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal and atherosclerotic human arteries. Cardiovasc Res. 1996;32:557–569. [PubMed] [Google Scholar]
- 29.Qing J, Du X, Chen Y, Chan P, Li H, Wu P, Marsters S, Stawicki S, Tien J, Totpal K, Ross S, Stinson S, Dornan D, French D, Wang QR, Stephan JP, Wu Y, Wiesmann C, Ashkenazi A. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J Clin Invest. 2009;119:1216–1229. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Lee BH, Williams IR, Kutok JL, Mitsiades CS, Duclos N, Cohen S, Adelsperger J, Okabe R, Coburn A, Moore S, Huntly BJ, Fabbro D, Anderson KC, Griffin JD, Gilliland DG. FGFR3 as a therapeutic target of the small molecule inhibitor PKC412 in hematopoietic malignancies. Oncogene. 2005;24:8259–8267. doi: 10.1038/sj.onc.1208989. [DOI] [PubMed] [Google Scholar]
- 31.Guglin M, Hartlage G, Reynolds C, Chen R, Patel V. Trastuzumab-induced cardiomyopathy: not as benign as it looks? A retrospective study. J Card Fail. 2009;15:651–657. doi: 10.1016/j.cardfail.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Mellor HR, Bell AR, Valentin JP, Roberts RR. Cardiotoxicity associated with targeting kinase pathways in cancer. Toxicol Sci. 2011;120:14–32. doi: 10.1093/toxsci/kfq378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


