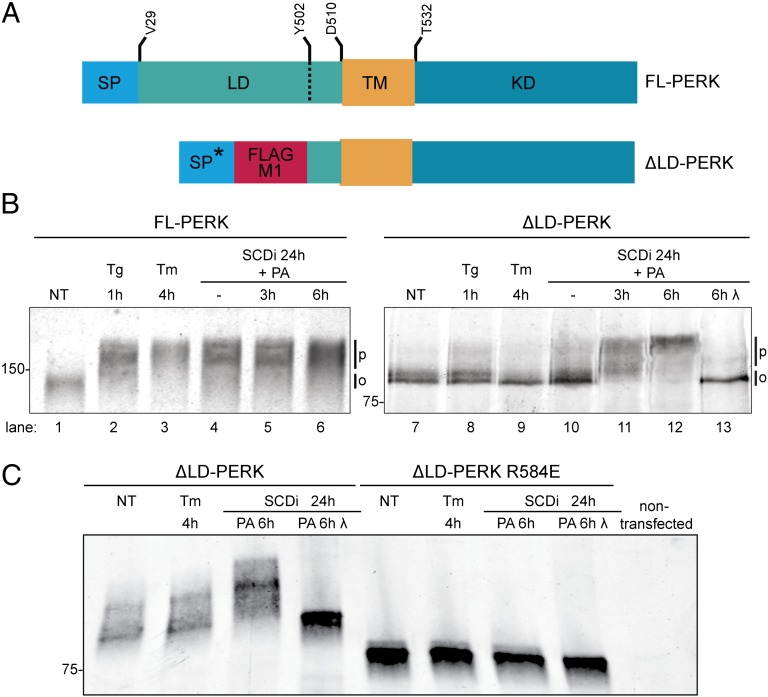
Fig. 2.
Luminal unfolded protein stress-sensing domain is dispensable to PERK activation by lipid saturation. (A) Schema of the PERK (FL) and the Flag-M1–tagged ER-targeted luminal domain-deleted (ΔLD) PERK proteins. The heterologous signal peptide of the ∆LD-PERK (SP*) whose cleavage exposes the Flag M1 tag, luminal (LD), transmembrane (TM), and kinase (KD) domains are noted. Residue numbering is based on UniProt Q9Z2B5. (B) Immunoblot of PERK immunopurified from wild-type mouse fibroblasts (FL-PERK) or PERK−/− cells transduced with ΔLD-PERK. Cells were exposed to thapsigargin (Tg; 1 µM), tunicamycin (Tm; 5 µg/mL) for the indicated times, or an SCD1 inhibitor for 24 h followed, where indicated, by palmitic acid (PA, 0.5 mM). The position of hypophosphorylated (0) and phosphorylated (P) PERK is indicated. λ-Phosphatase was applied in vitro to the purified protein (lane 13). (C) Immunoblot of wild-type and R584E dimerization mutant ∆LD-PERK immunopurified from transfected HEK293T cells treated as in B.