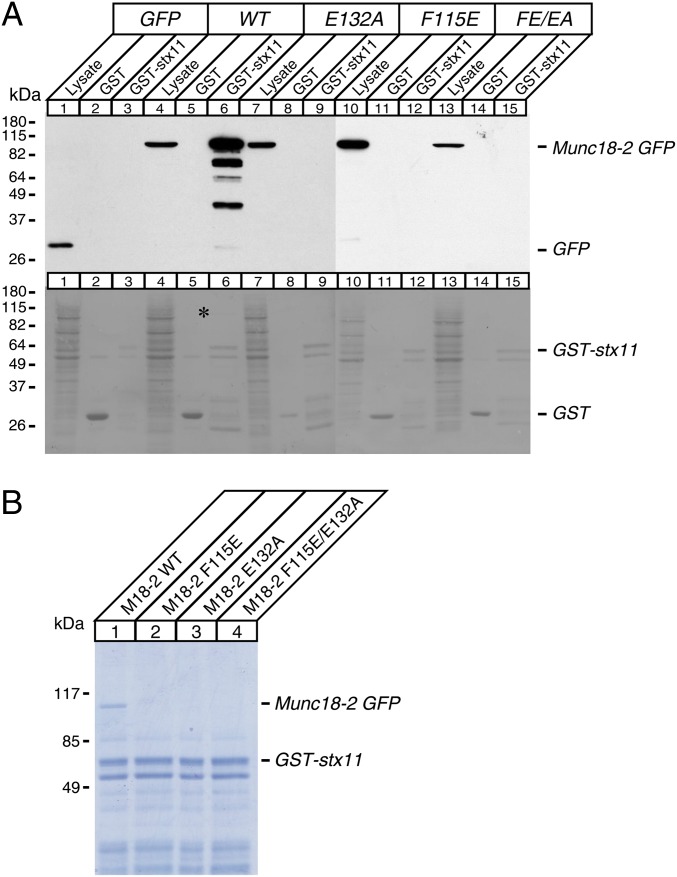
Fig. 5.
Hydrophobic pocket mutants of Munc18-2 abolish the direct interaction with syntaxin-11. (A, Upper) Solubilized lysates of HEK-293FT cells that were transfected with EmGFP, wild-type Munc18-2-EmGFP, F115E, E132A, and F115E/E132A of Munc18-2-EmGFP were incubated with 10 μg GST or GST-syntaxin-11 attached to glutathione agarose beads. The mixtures were washed extensively, and the proteins were analyzed on SDS/PAGE and immunoblotting, using mouse monoclonal anti-EmGFP antibody. Solubilized lysates of HEK-293FT cells were loaded in lysate lanes. (A, Lower) Ponceau S staining of the above membrane showing similar loadings between lanes. *Wild-type Munc18-2-EmGFP band that was pulled down by GST-syntaxin-11. (B) The binding mixture between solubilized lysates of HEK-293FT cells that were transfected with wild-type Munc18-2-EmGFP, F115E, E132A, F115E/E132A, and 10 μg of GST-syntaxin-11 was analyzed on SDS/PAGE, followed by Coomassie Blue staining. Note that only wild-type Munc18-2-EmGFP was pulled down by GST-syntaxin-11, not any of the mutants.