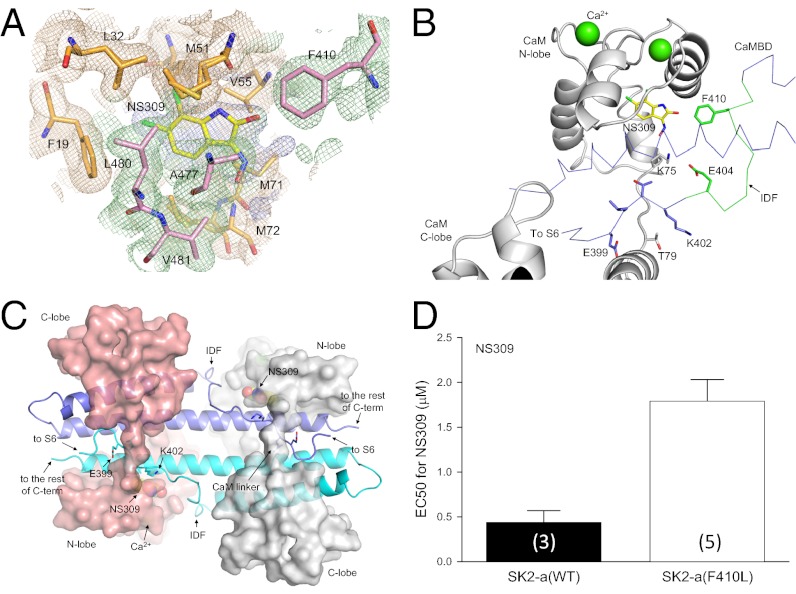
Fig. 3.
Transition of the IDF conformation from unstructured to structured in the presence of NS309, a potent channel modulator. (A) Electron density map (light blue) shows the presence of additional electron density at the interface between CaM (wheat) and the CaMBD (pale green) at the CaM N-lobe. The map is contoured at 0.5 σ and is overlaid with the current refined coordinates for NS309 and amino acid residues from CaM (orange) and the CaMBD (pink) that surround NS309. (B) F410 from the IDF is part of the NS309 binding pocket. The distance between F410 and NS309 is 3.4 Å. Note the appearance of the IDF at a different viewing angle. Without NS309, the conformation of F410 cannot be determined. (C) Structure of the entire 2 × 2 CaM–CaMBD2-a complex in the presence of NS309. The structure model has the same view angle as in Fig. 1B. The most noticeable difference between this structure and that of Fig. 1B is the appearance of the well-defined IDF structure. (D) The F410L mutation reduces the modulatory effects of NS309. Values in parentheses indicate the number of experiments.