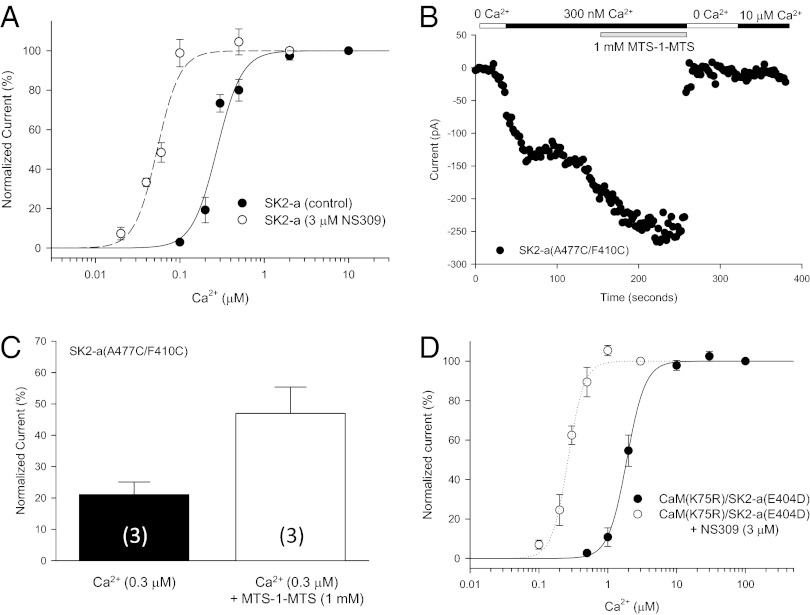
Fig. 4.
The IDF on Ca2+-dependent activation of SK channels. (A) NS309 increases the open probability of SK2 at a given Ca2+ concentration. Dose–response curves of Ca2+-dependent activation of SK2-a were obtained with or without 3 μM NS309 in the bath solution. (B) Application of the cross-linker MTS-1-MTS (1 mM) doubles the current size of the same patch in the presence of 300 nM Ca2+. After the bath solution was switched to 0 Ca2+, subsequent application of 10 μM Ca2+ no longer could activate the channels modified by MTS-1-MTS, indicating that cross-linking has changed the structure of the IDF of the SK channels irreversibly. (C) Cross-linking A477C of the CaMBD and F410C of the IDF mimics the effect of NS309. The membrane patches expressing A477C/F410C were perfused sequentially with a solution of 10 μM Ca2+, 300 nM Ca2+, and 300 nM Ca2+ plus 1 mM MTS-1-MTS. The current amplitudes were normalized to that at 10 μM Ca2+. Values in parentheses indicate the number of experiments. (D) Effects of mutations on the salt bridge between K75 of CaM and E404 of the IDF. The mutant pair K75R:E404D was able to rescue activation of the mutant channel by Ca2+. NS309 is able to potentiate the Ca2+-dependent activation of the mutant pair.