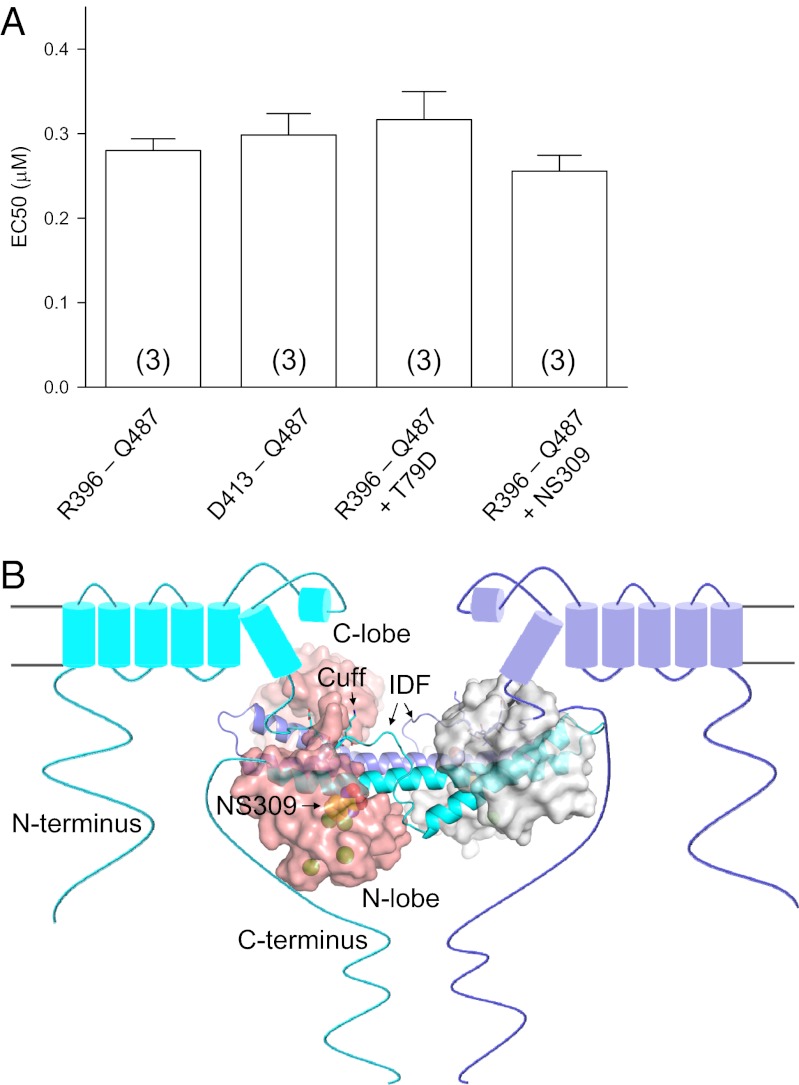
Fig. 5.
The IDF does not alter the affinity of CaM for Ca2+. (A) Effects of the IDF, NS309, or T79D on the Ca2+-dependent formation of the CaM–CaMBD2-a complex. Fluorophore-labeled CaM(T34C) was mixed with channel peptides R396–Q487 (cuff + IDF + CaMBD2-a) or D413–Q487 (CaMBD) at different Ca2+ concentrations. A CaM mutant, CaM(T34C/T79D), or NS309 (saturating) also was used for the binding assays as indicated. No changes were seen in the apparent affinity for Ca2+ in Ca2+-dependent formation of the CaM–CaMBD2-a complex under these manipulations. Values in parentheses indicate the number of experiments. (B) Structural model depicting the location of the CaM–CaMBD complex and the appearance of the IDF by NS309. Because of the interaction between the channel cuff and the CaM linker, the CaM–CaMBD complex is located much closer to the plasma membrane than previously thought. The unique conformation of the IDF, induced by NS309, couples the Ca2+ binding to CaM and the mechanical opening of the channel pore and increases the channel open probability at a given Ca2+ concentration.