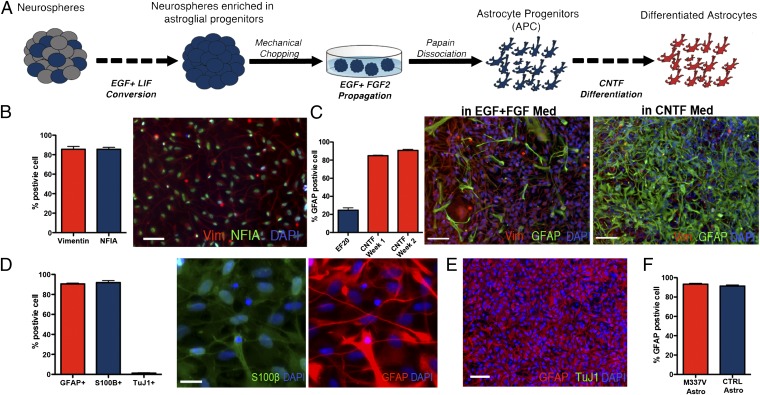
Fig. 1.
Generation of astrocytes from iPSC lines. (A) iPSC-derived neurospheres were enriched for APCs by culturing in EGF/LIF-containing medium for 2–4 wk and then expanded in EGF/FGF2-containing medium. To obtain monolayer cultures, enriched neurospheres were dissociated by using papain and differentiated with CNTF. (B) APCs in monolayer cultures maintained a homogenous identity and morphology, featuring a high percentage of cells positive for the glial progenitors markers Vimentin (85.6 ± 2.9% SEM) and NFIA (79.8 ± 1.9% SEM; scale bar: 100 µm.) (C) APC presented 24.6 ± 2.6% SEM cells positive for GFAP during proliferation in EGF and FGF2; this percentage increased rapidly during the first 7 d of CNTF differentiation (84.9 ± 0.5% SEM), reaching a peak after 14 d (90.8 ± 1.1% SEM; scale bar: 100 μm). (D) Differentiation of monolayer APCs for 14 d in CNTF resulted in a population of astrocytes positive for GFAP (90.6 ± 0.7% SEM) and S100β (91.9 ± 1.9% SEM) (images represent the same field; scale bar: 25 µm.) A few neurons were found in the differentiated cultures (1.7 ± 0.4% SEM). (E) Representative field for a differentiated iPSC-derived astrocytes after 14 d of CNTF differentiation, labeled for GFAP (red) and TuJ1 (green), showing that the protocol gives rise to highly enriched cultures of astrocytes. (Scale bar: 100 μm). (F) M337V and CTRL iPSC lines showed comparable numbers of GFAP-positive cells postdifferentiation (M337V, 92.3 ± 1.0%; WT, 91.36 ± 1.0%; SEM).