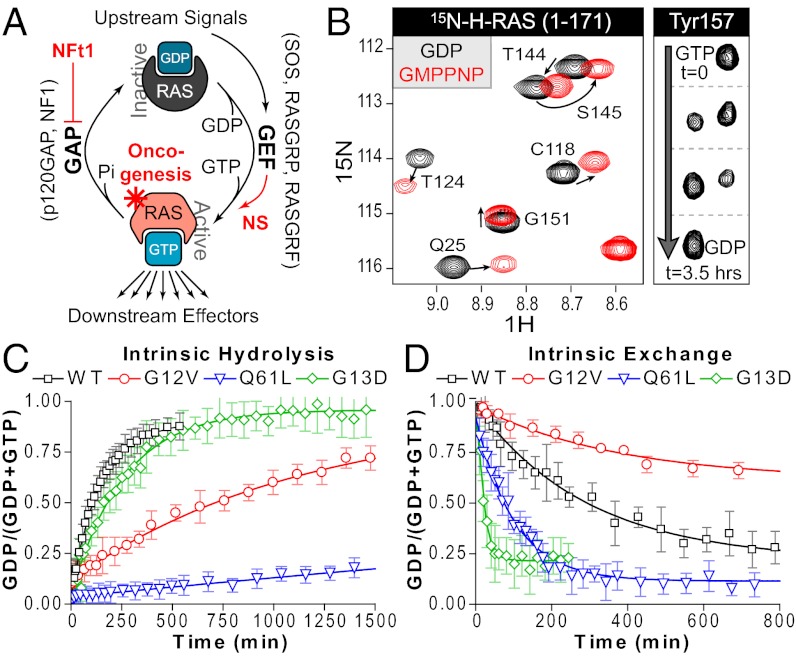
Fig. 1.
Monitoring GTPase activity by NMR. (A) RAS signaling revolves around the GTPase cycle, whereby RAS is activated when GDP is exchanged for GTP, a process facilitated by GEFs. Slow intrinsic hydrolysis is enhanced by the activity of GAPs. Disease states can occur by several mechanisms, including loss of NF1 GAP activity (NFt1, Neurofibromatosis type 1), increased GEF activity, or RAS mutation. (B) NMR-based analysis of nucleotide-dependent changes in RAS conformation. Overlay of 2D 1H/15N HSQC spectra showing select amide resonances of RAS bound to GDP (black) or GMPPNP (red). RAS-GTP-derived peaks eventually shift to GDP positions as hydrolysis occurs (shown for Y157). (C) Use of peak intensity data to determine rates of intrinsic hydrolysis for wild-type and mutant RAS. 15N-RAS proteins were loaded with GTP and hydrolysis monitored by sequential HSQCs. (D) Monitoring exchange reactions of wild-type and mutant RAS. GDP-bound RAS intrinsically exchanges in 10-fold molar excess GTPγS. Also see Fig. S1 and Table S2.