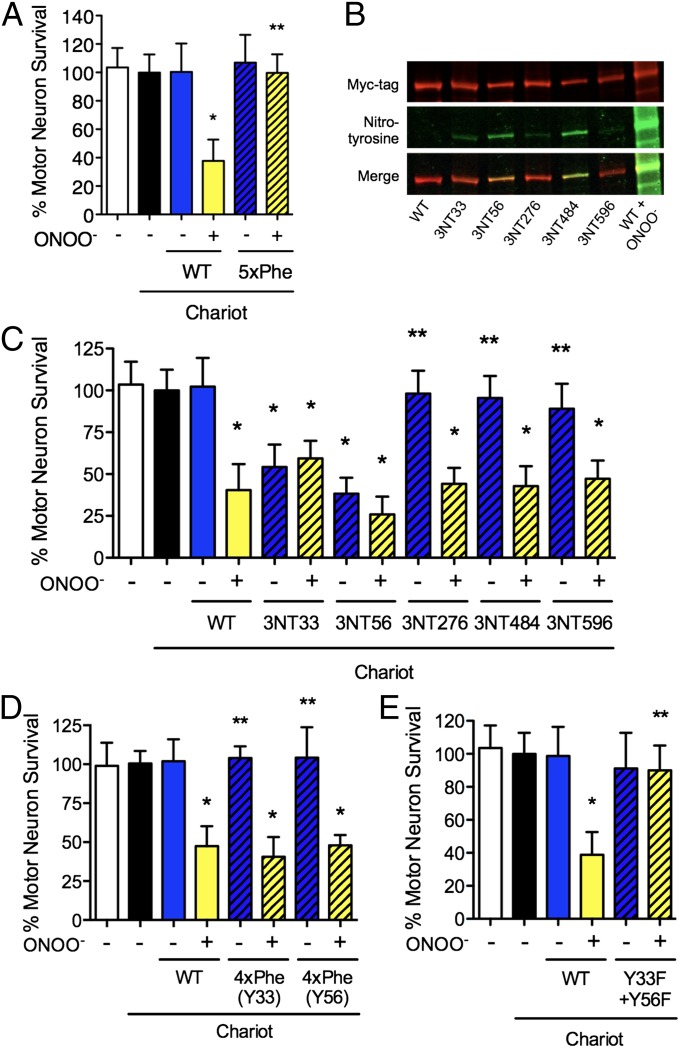
Fig. 4.
Nitration of a single tyrosine residue of Hsp90 is sufficient to induce cell death. (A) The replacement of the tyrosine residues prone to nitration on Hsp90 (WT) by phenylalanine (5xPhe) prevented peroxynitrite-treated Hsp90 toxicity. (B) The presence of nitrotyrosine (3NT) in the recombinant proteins carrying a single nitrated residue was assayed by Western blot probed with antibodies against myc-tag (green) and nitrotyrosine (red). The merged image is shown in yellow. (C) Assay for the toxicity of Hsp90 with nitrotyrosine at position 33, 56, 276, 484, or 596 with (yellow bars) or without (blue bars) peroxynitrite treatment. Nitrotyrosine at positions 33 or 56 was sufficient to induce motor neuron death. (D and E) The relevance of tyrosine nitration was confirmed by directed mutagenesis of the relevant residues. Peroxynitrite-treated Hsp90 remained toxic with tyrosine only at position 33 or 56 and the other four residues replaced by phenylalanine [4xPhe(Y33) and 4xPhe(Y56)] (D), whereas replacement of residues 33 and 56 of Hsp90β by phenylalanine (Y33F + Y56F) prevented toxicity induced by peroxynitrite treatment (E). *P < 0.05 versus WT, **P < 0.05 versus WT + ONOO− by ANOVA followed by Bonferroni multiple comparison test.