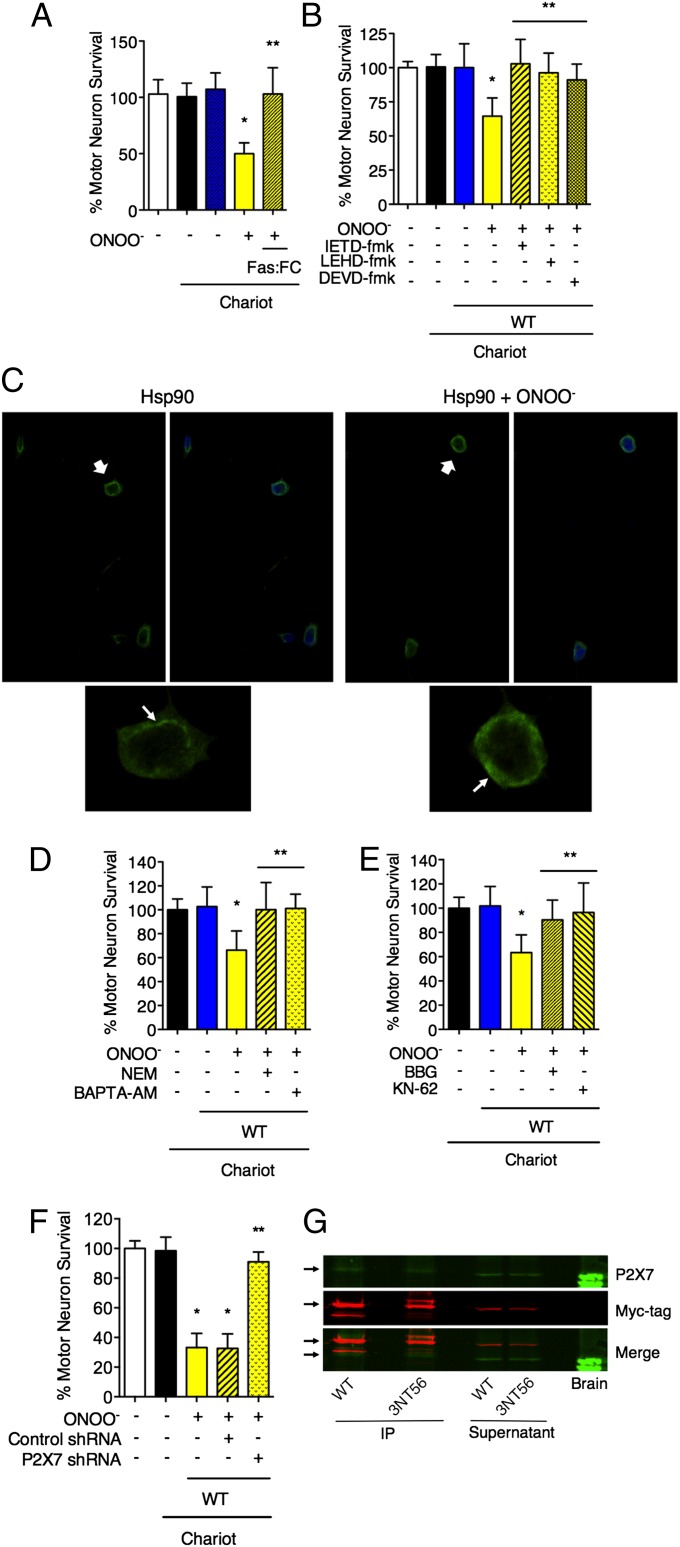
Fig. 5.
Nitrated Hsp90 induces cell death through the activation of the P2X7 receptor that leads to exposure of FasL on the cellular membrane and the activation of the Fas pathway. (A) Incubation with the FasL decoy Fas:FC (1 μg/mL) for 24 h completely protected motor neurons from nitrated Hsp90-induced cell death. (B) Motor neurons were incubated for 24 h with inhibitors of caspase 8 (IEDT-fmk; 10 μM), 9 (LEHD-fmk; 20 μM), and 3 (DEVD-fmk; 20 μM) after the intracellular delivery of peroxynitrite-treated Hsp90. *P < 0.05 versus WT, **P < 0.05 versus WT + ONOO− by ANOVA followed by Bonferroni multiple comparison test. (C) Motor neurons show mobilization of FasL to the plasma membrane upon treatment with peroxynitrite-treated Hsp90. The motor neurons were incubated for 16 h after the intracellular delivery of unmodified (Hsp90) or peroxynitrite-treated Hsp90 (Hsp90 + ONOO−). The cells then were stained for FasL (green). The cell nuclei are stained in blue. (Upper) The white arrows in indicate the cells that are shown magnified in the lower panels. (Lower) The white arrows indicate the cellular location of FasL signal. See also Fig. S3. (D) The motor neurons were cultured in the presence of NEM (2 μM) or the intracellular calcium chelator BAPTA-AM (5 μM) for 24 h after the intracellular delivery of peroxynitrite-treated Hsp90. The inhibition of the last steps of exocytosis by NEM and the chelation of intracellular calcium by BAPTA-AM completely protected motor neurons from peroxynitrite-treated Hsp90-induced cell death. (E and F) Both the inhibition of the purinergic receptor P2X7 by BBG (10 μM) and KN-62 (1 μM) (E) and the receptor knockdown by lentiviral particles expressing P2X7 shRNA (F) prevented motor neuron death induced by peroxynitrite-treated Hsp90. Motor neurons were transduced with lentiviral particles expressing P2X7 or control shRNA for 84 h before the intracellular delivery of nitrated Hsp90. *P < 0.05 versus Chariot, **P < 0.05 versus WT + ONOO− by ANOVA followed by Bonferroni multiple comparison test. (G) Nitrated Hsp90 coimmunoprecipitated with the P2X7 receptor. Homogenates from PC12 were incubated for 1 h with Hsp90β-myc (WT) or with Hsp90β-myc nitrated at position 56 (3NT56), and the recombinant proteins were immunoprecipitated with an anti-myc antibody before SDS/PAGE. The membranes then were blotted for P2X7 (green) and the myc-tag (red). Brain homogenate was used as a positive control for P2X7.