Fig. 2.
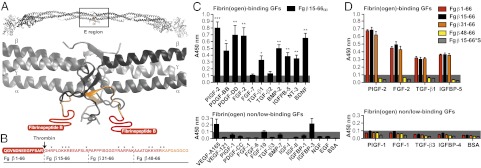
GF binding to the heparin-binding domain of fibrin(ogen). (A) Ribbon diagram representation of human fibrinogen and its central region E (generated using Swiss-PdbViewer). The coiled-coil domains are in gray; the N terminus portions of the Bβ chain are in yellow (Fg β59–66), and the N terminus parts of the Bβ chain missing in the crystal structure are in red (Fg β1–58). Fibrinopeptide B is highlighted in red (Fg β1–14). (B) Amino acid sequences of the fibrin(ogen) fragments (3). The region within Fg β1–66 with unknown tertiary structure is in red, and the known tertiary structure is in orange. The arrow indicates the thrombin cleavage site to remove fibrinopeptide B, and the stars indicate lysine and arginine residues. (C and D) GF binding to fibrinogen fragments. ELISA plates were coated with GFs or BSA and further incubated with fibrin(ogen) fragments. Bound fibrin(ogen) fragments were detected using an antibody against the tag (n ≥ 4; mean ± SEM). A signal significantly greater than 0.1 (gray box) was considered representative of a relevant binding. For D: *P < 0.05; **P < 0.01; ***P < 0.001, one-sample Student t test.
