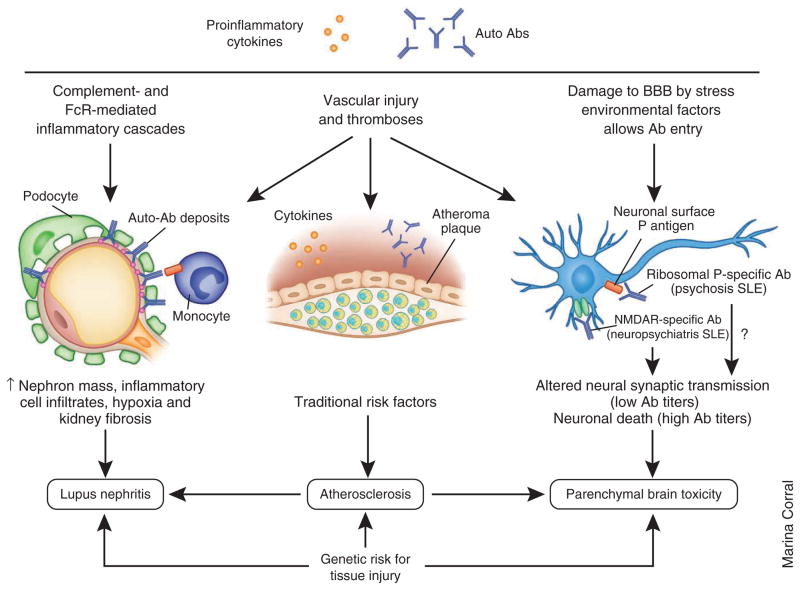
Figure 4.
Mechanisms for organ damage. Organ damage is caused by immune activation and inflammation but is also influenced by genetic and nonimmunologic environmental factors. Autoantibodies and circulating inflammatory mediators trigger tissue injury in target organs by a variety of mechanisms. In the kidneys, immune complex deposition induces complement- and FcR-mediated inflammatory cascades that lead to the activation or injury of renal resident cells, which in turn release inflammatory mediators, leading to the recruitment of inflammatory cells. Long term renal damage is caused by ongoing inflammation, vascular injury by systemic and local mediators, hypoxia and fibrosis. Nephritis occurs in approximately 50% of adult and 80% of pediatric patients with SLE, and the rate of end-stage renal disease in the United States caused by SLE seems to be increasing, especially in minority patients. In the cardiovascular system, autoantibodies and soluble inflammatory mediators cause vascular endothelial injury by inducing endothelial apoptosis or activation. Recruitment of monocytes to the injured site is also crucial for plaque formation. Other proatherogenic factors in addition to traditional risk factors include oxidized low-density lipoproteins, antibodies to oxidized lipids and proinflammatory high-density lipoproteins. The 10-year risk for a coronary event or stroke is 7.5- to 17-fold increased in patients with SLE compared with healthy individuals. In the central nervous system, autoantibodies access the brain when the BBB is attenuated by inflammatory mediators or environmental factors, such as cigarette smoke or neurotransmitters released by stress. Once deposited in the brain, autoantibodies may induce neuron apoptosis or alter neuronal synaptic transmission. Neurologic injury can also result from secondary causes, such as thrombosis or infections. All these pathogenic changes lead to a wide range of neuropsychiatric manifestations of SLE, including progressive cognitive dysfunction. Neuropsychiatric manifestations may occur relatively early in the disease process, and they affect up to 40% of patients.