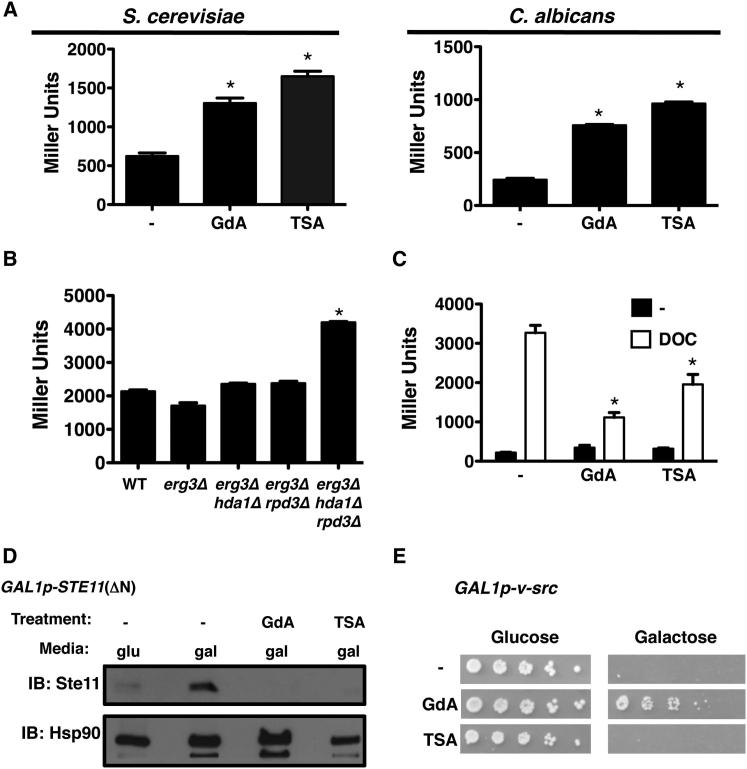
Figure 6.
Inhibition of KDACs Impairs Hsp90 Client Protein Activation and Stability
(A) Geldanamycin (GdA) and trichostatin A (TSA) induce the heat shock response in S. cerevisiae and C. albicans. Log phase cultures of strains with an HSE-lacZ reporter (S. cerevisiae) or HSP70p-lacZ reporter (C. albicans) were untreated (−) or treated with GdA or TSA. β-Galactosidase data are mean ± SD for technical triplicates and are representative of biological duplicates.
(B) Deletion of HDA1 and RPD3 enhances the heat shock response. Log phase S. cerevisiae harboring an HSE-lacZ reporter was grown at 42°C for 3 hr.
(C) TSA blocks glucocorticoid receptor (GR) maturation in S. cerevisiae. Wild-type S. cerevisiae containing a GR expression plasmid and a GR reporter plasmid with a GR response element (GRE) driving lacZ expression was used. Maturation of GR upon addition of deoxycorticosterone (DOC, 10 μM) was assessed in the absence and presence of GdA or TSA.
(D) TSA leads to destabilization of Ste11. Wild-type S. cerevisiae was grown in SD with glucose (glu) as a negative control or galactose (gal) to induce 6×His-Ste11 expression. Cultures grown in galactose were grown without inhibitor (−) or with GdA or TSA. Total protein was resolved by SDS-PAGE, and blots were hybridized with α-His or with α-Hsp90 as a loading control.
(E) TSA does not rescue v-Src-induced lethality in S. cerevisiae. S. cerevisiae containing a vector with v-Src under the control of a galactose-inducible promoter was spotted in 5-fold dilutions onto SD with 2% glucose or 2% galactose with no drug (−), GdA, or TSA. Images are representative of biological duplicates.
∗p < 0.001.