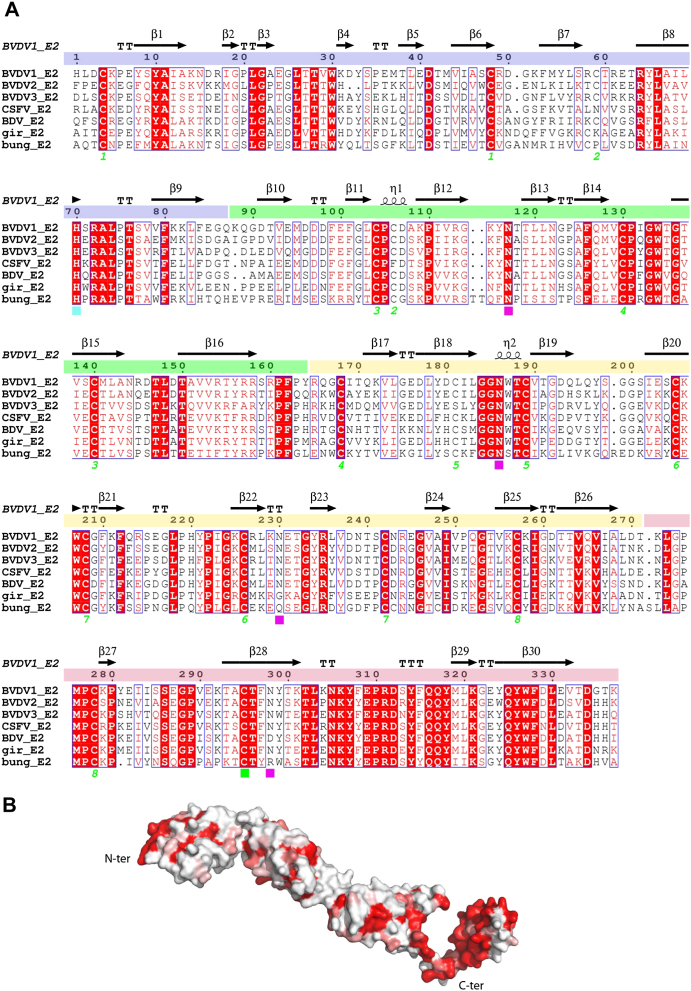
Figure S1.
Amino Acid Conservation between Pestiviruses E2 Glycoprotein, Related to Figures 1, 2, and 3
(A) Sequence alignment of BVDV1 E2 with E2 proteins from other pestiviruses: BVDV2, BVDV3, Classical Swine Fever virus (CSFV), Border Disease virus (BDV), Pestivirus of giraffe (gir) and Bungowannah virus (bung). Conserved residues are drawn in red boxes, similar residues in red type. The secondary structure assignment for BVDV1 E2 is shown at the top and domains DA, DB, DC and DD are colored in blue, green, yellow and red, respectively. Cysteines involved in intra-chain disulfide bridges are marked by a green number, the single cysteine involved in the inter-chain disulfide bridge is marked by a green square, the four glycosylation sites of BVDV1 E2 are marked with magenta squares and the conserved histidine is marked with a cyan square.
(B) Surface representation of a monomer of BVDV E2 colored according to sequence conservation from white (non-conserved) to red (conserved).