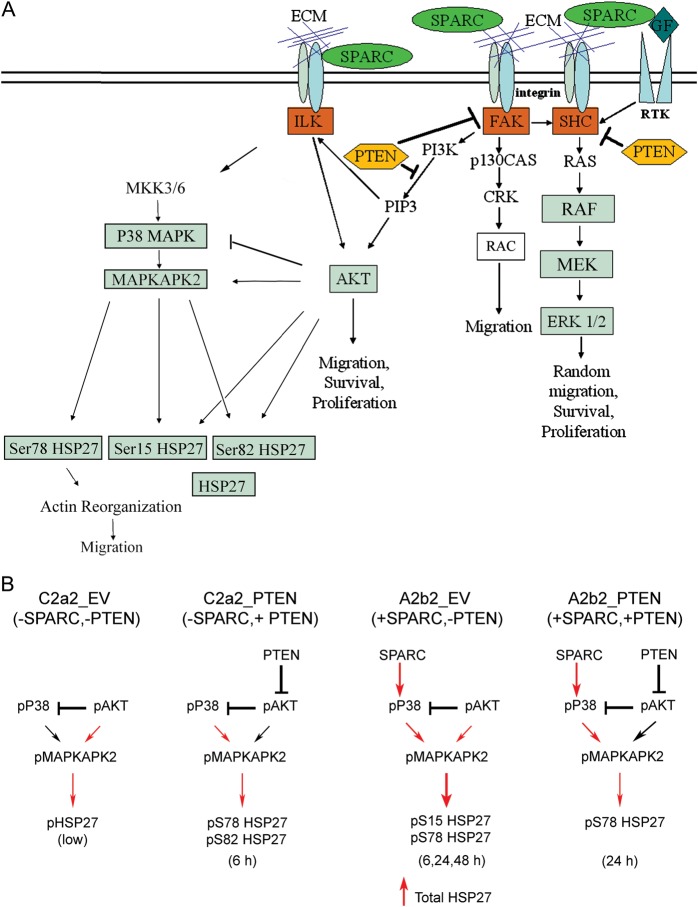
Fig. 6.
Proposed signaling mechanism for SPARC ± PTEN. (A) Summary of SPARC-mediated signaling pathways that impact glioma migration. We have previously demonstrated that SPARC induces glioma migration. This migration is accompanied by increases in pP38 MAPK and pHSP27 and can be suppressed with the inhibition of HSP27 expression.16 In addition, we have demonstrated that PTEN suppresses pAkt, and together with SPARC, suppresses the Raf-MEK-ERK signaling pathway.17 PTEN also inhibits SPARC-induced migration.17 Data presented in this manuscript show that PTEN suppresses pAkt, which correlates with suppression of pMAPKAPK2, which in turn limits the increase in phosphorylation of Ser78 relative to total HSP27. This decrease in pSer78 HSP27/total HSP27 correlates with suppressed migration. (B) Proposed mechanisms for SPARC and PTEN regulation of HSP27 expression and phosphorylation. In the absence of SPARC and PTEN (C2a2_EV), pAkt inhibits P38 MAPK but activates a low level of pHSP27 via direct activation of pMAPKAPK2. In the absence of SPARC, PTEN (C2a2_PTEN) inhibits Akt activation, thereby suppressing the inhibition of pP38 MAPK, which activates MAPKAPK2 to phosphorylate Ser78 and Ser82 of HSP27, but only at 6 h. In the absence of PTEN, SPARC (A2b2_EV) upregulates pP38 MAPK, which activates MAPKAPK2 to enhance Ser15 HSP27 at 6 h and sustained Ser78 HSP27 at 6, 24, and 48 h. SPARC also increases total HSP27 expression. PTEN in the presence of SPARC (A2b2_PTEN) suppresses pAkt, which limits the activation of SPARC-induced MAPKAPK2, thereby suppressing SPARC-induced pHSP27. We propose that the overall increase in pSer78 HSP27 relative to total HSP27 in SPARC-expressing cells promotes uncapping of unphosphorylated HSP27 from actin filaments to promote actin polymerization that promotes migration, whereas PTEN suppresses these SPARC-induced changes. Red arrows: positive signaling; black arrows: suppressed signaling. Abbreviation: ECM, extracellular matrix.