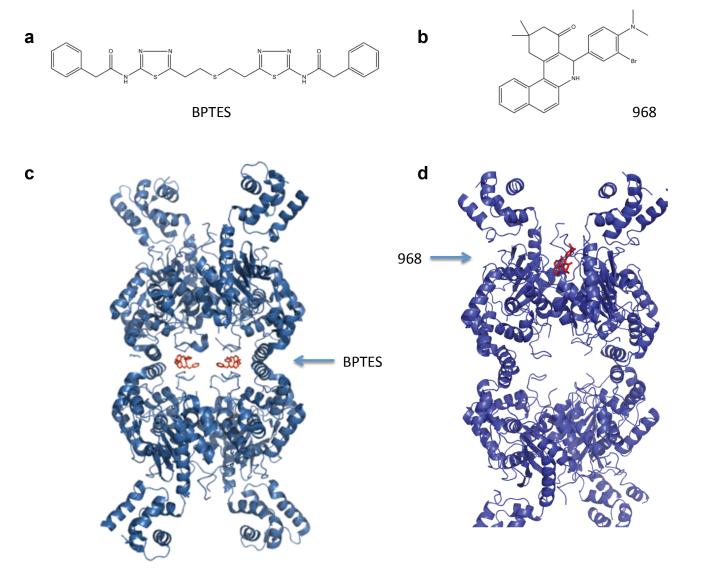
Figure 3.
Comparison of two GLS1 allosteric inhibitors, BPTES and 968. a. Chemical structure of BPTES. b. Chemical structure of 968. c. X-ray crystallographic structure of human GLS1 bound to BPTES, adapted from [35,36]. Two BPTES molecules are bound at the dimer–dimer interface of the GLS1 tetramer. d. Molecular docking model of 968 to GAC, adapted from [37]. One molecule of 968 is proposed to bind into a hydrophobic pocket formed by the N- and C-termini at the monomer–monomer interface of the GAC dimer.