Table 3.
Ruthenium catalyzed [4+2] cycloaddition of isoprene 4b with vicinally dioxygenated hydrocarbons 1a-1d, 1f, 2a-2d, 2f, and 3a-3d, 3f.a
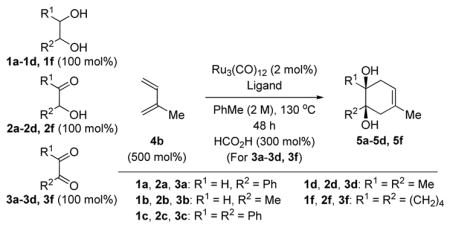
| |||
|---|---|---|---|
| Entry | Cycloadduct | Reactant | Yield % |
| 1 |
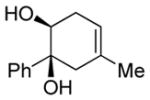
|
Diol 1a | 78b |
| Hydroxyketone 2a | 75b | ||
| Dicarbonyl 3a | Trace | ||
| 2 |
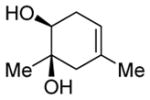
|
Diol 1b | 60b,e |
| Hydroxyketone 2b | 72b | ||
| Dicarbonyl 3b | Trace | ||
| 3 |
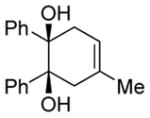
|
Diol 1c | 90c |
| Hydroxyketone 2c | 98c | ||
| Dicarbonyl 3c | 70d | ||
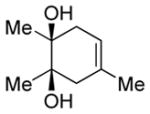
| 4 |
|
Diol 1d | 84b |
| Hydroxyketone 2d | 61b | ||
| Dicarbonyl 3d | 35d,f | ||
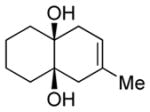
| 5 |
|
Diol 1f | 90b |
| Hydroxyketone 2f | 68b | ||
| Dicarbonyl 3f | 54d,f | ||
Yields are of material isolated by silica gel chromatography.
PCy3 (12 mol%).
BIPHEP (6 mol%).
RuH2CO(PPh3)3 (6 mol%), BIPHEP (6 mol%).
150 °C.
HCO2H (300 mol%). See Supporting Information for further details and structural assignments.
