Figure 2.
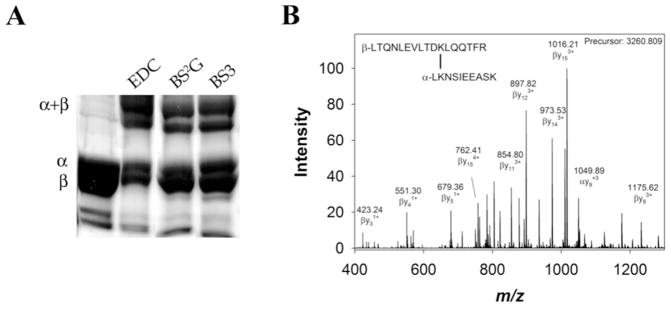
Representative cross-linking results. A) Cross-linked Ms sGC NT13, examined by SDS-PAGE and stained with Coomassie brilliant blue. Individual bands were cut from the gel, digested with trypsin and examined by tandem mass spectrometry. B) Representative cross- linked peptide MS/MS spectrum. BS2G cross-link α1 K434 to β1 K366 was found in the +4 charge state due to two amine termini and lysine/arginine at the trypsin cleavage sites, and displayed a 22 ppm error in observed vs. calculated precursor mass (Table 1). The cross-linked peptide was observed and selected for fragmentation in 7 scans in a single experiment. Between 7 and 36 fragments were identified in each scan, each with a mass accuracy of approximately 1 ppm. Representative fragments are labeled in the mass spectrum. Identified fragments in this spectrum included β1 fragments b4–10, y2–6, y8–15 (which contain the cross-linker and entire α1 peptide), α1 fragments y2–8, and α1 fragments b3–5 (which contain the cross-linker and entire β1 peptide).
