Figure 10.
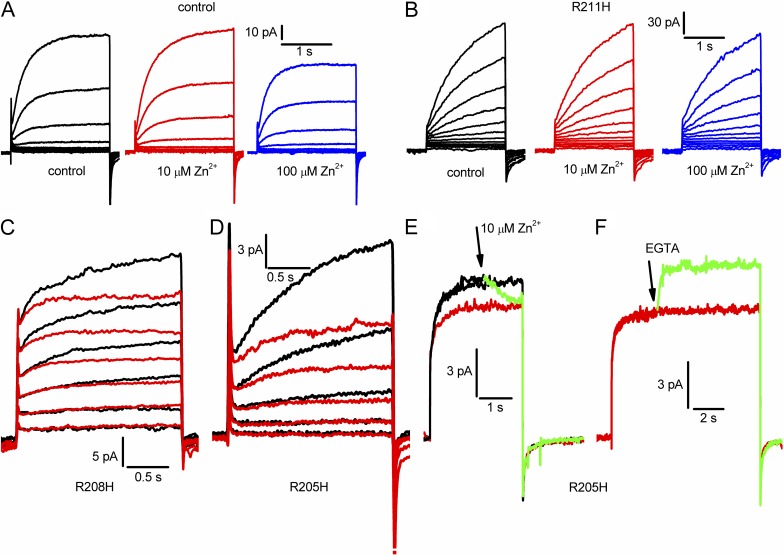
Evaluation of external accessibility of the three Arg residues in the S4 helix of hHV1. All measurements were made in a construct designed to have low sensitivity to Zn2+, H140A/H193A/K221stop. (A) Currents during identical families of pulses in 10-mV increments up to +60 mV in the background construct in whole-cell configuration, in the presence of 0, 10, or 100 µM Zn2+ in the bath solution. (B) Whole-cell currents in the R211H mutant during identical families of pulses in 10-mV increments up to +80 mV, in the presence of 0, 10, or 100 µM Zn2+. (C) Currents during identical pulse families in 20-mV increments up to +60 mV in the R208H mutant in the absence (black) or presence of 10 µM Zn2+ (red). (D) Currents during identical pulse families in 10-mV increments up to +30 mV in the R205H mutant in the absence (black) or presence of 10 µM Zn2+ (red). (E) Currents during three consecutive pulses to +90 mV in a cell transfected with R205H. At or slightly before the arrow, 10 µM Zn2+ was applied to the bath, reducing the current during the pulse (green). The next pulse (red) shows the steady-state extent of Zn2+ inhibition. (F) Later in the same experiment, EGTA was added during a pulse at or before the arrow, rapidly relieving Zn2+ effects. For all parts, pHo was 7.0; pHi was 7.0 for A, B, E, and F; and pHi was 5.5 for C and D.