Abstract
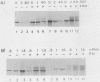
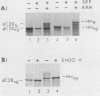
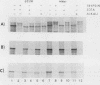
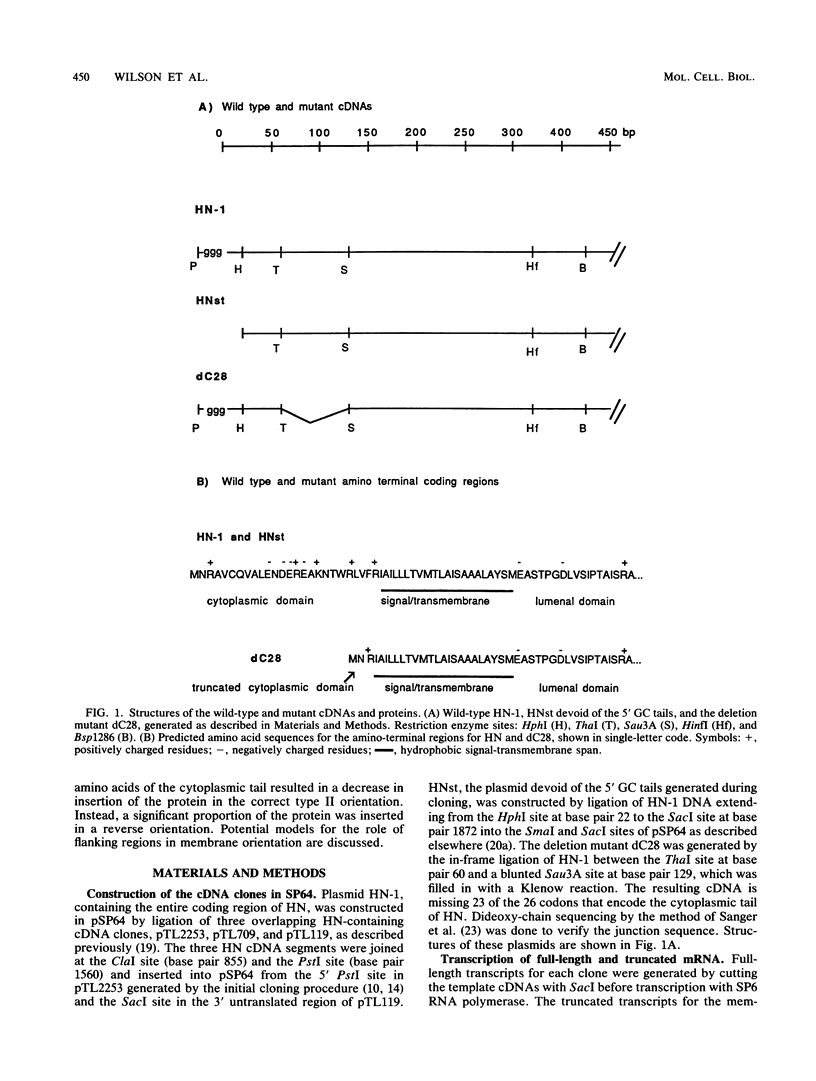
The hemagglutinin-neuraminidase (HN) protein of Newcastle disease virus (NDV) is a type II glycoprotein oriented in the plasma membrane with its amino terminus in the cytoplasm and its carboxy terminus external to the cell. We have previously shown that the membrane insertion of HN protein requires signal recognition particle SRP, occurs cotranslationally, and utilizes the same GTP-dependent step that has been described for secretory proteins, type I proteins, and multispanning proteins (C. Wilson, R. Gilmore, and T. Morrison, Mol. Cell. Biol. 7:1386-1392, 1987; C. Wilson, T. Connolly, T. Morrison, and R. Gilmore, J. Cell Biol. 107:69-77, 1988). The role of the amino-terminal cytoplasmic domain in the faithful membrane insertion of this type II protein was explored by characterizing the membrane integration of a mutant lacking 23 of the 26 amino acids of the cytoplasmic domain. The mutant protein was able to interact with SRP, resulting in translation inhibition, membrane targeting, and membrane translocation, but the efficiency of translocation was considerably lower than for the wild-type HN protein. In addition, a significant proportion of the mutant protein synthesized in the presence of SRP and microsomal membranes was associated with the membrane in an EDTA- and alkali-insensitive manner yet integrated into membranes with its carboxy-terminal domain on the cytoplasmic side of membrane vesicles. Membrane-integrated molecules with this reverse orientation were not detected when the mutant protein was synthesized in the absence of SRP or a functional SRP receptor. Truncated mRNAs encoding amino-terminal segments of the wild-type and mutant proteins were translated to prepare ribosomes bearing arrested nascent chains. The arrested mutant nascent chain, in contrast to the wild-type nascent chain, was also able to insert into membranes in a GTP- and SRP-independent manner. Results suggest that the cytoplasmic domain plays a role in the proper membrane insertion of this type II glycoprotein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. J., Hogue B. G., Nayak D. P. Redundancy of signal and anchor functions in the NH2-terminal uncharged region of influenza virus neuraminidase, a class II membrane glycoprotein. J Virol. 1988 Oct;62(10):3824–3831. doi: 10.1128/jvi.62.10.3824-3831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. Formation of a functional ribosome-membrane junction during translocation requires the participation of a GTP-binding protein. J Cell Biol. 1986 Dec;103(6 Pt 1):2253–2261. doi: 10.1083/jcb.103.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell. 1989 May 19;57(4):599–610. doi: 10.1016/0092-8674(89)90129-3. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Blobel G. Transient involvement of signal recognition particle and its receptor in the microsomal membrane prior to protein translocation. Cell. 1983 Dec;35(3 Pt 2):677–685. doi: 10.1016/0092-8674(83)90100-9. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Flint N., Gough N. M., Dobberstein B. A tripartite structure of the signals that determine protein insertion into the endoplasmic reticulum membrane. J Cell Biol. 1989 Apr;108(4):1227–1236. doi: 10.1083/jcb.108.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K. E., Gilmore R. Guanosine triphosphate promotes the post-translational integration of opsin into the endoplasmic reticulum membrane. J Biol Chem. 1988 Mar 25;263(9):4381–4385. [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. Synthesis of ds-cDNA involving addition of dCMP tails to allow cloning of 5'-terminal m RNA sequences. Methods Enzymol. 1983;100:285–292. doi: 10.1016/0076-6879(83)00062-2. [DOI] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal and membrane anchor functions overlap in the type II membrane protein I gamma CAT. J Cell Biol. 1988 Jun;106(6):1813–1820. doi: 10.1083/jcb.106.6.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. The membrane-spanning segment of invariant chain (I gamma) contains a potentially cleavable signal sequence. Cell. 1986 Sep 26;46(7):1103–1112. doi: 10.1016/0092-8674(86)90710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoff L., Lin B. C., Sveda M. M., Lai C. J. Glycosylation and surface expression of the influenza virus neuraminidase requires the N-terminal hydrophobic region. Mol Cell Biol. 1984 Jan;4(1):8–16. doi: 10.1128/mcb.4.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnes L. W., Wilde A., Morrison T. G. Nucleotide sequence of the gene encoding the Newcastle disease virus hemagglutinin-neuraminidase protein and comparisons of paramyxovirus hemagglutinin-neuraminidase protein sequences. Virus Res. 1987 May;7(3):187–202. doi: 10.1016/0168-1702(87)90027-x. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., McGinnes L. W. Avian cells expressing the Newcastle disease virus hemagglutinin-neuraminidase protein are resistant to Newcastle disease virus infection. Virology. 1989 Jul;171(1):10–17. doi: 10.1016/0042-6822(89)90505-9. [DOI] [PubMed] [Google Scholar]
- Nicchitta C. V., Blobel G. Nascent secretory chain binding and translocation are distinct processes: differentiation by chemical alkylation. J Cell Biol. 1989 Mar;108(3):789–795. doi: 10.1083/jcb.108.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perara E., Rothman R. E., Lingappa V. R. Uncoupling translocation from translation: implications for transport of proteins across membranes. Science. 1986 Apr 18;232(4748):348–352. doi: 10.1126/science.3961485. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Walter P. Each of the activities of signal recognition particle (SRP) is contained within a distinct domain: analysis of biochemical mutants of SRP. Cell. 1988 Jan 15;52(1):39–49. doi: 10.1016/0092-8674(88)90529-6. [DOI] [PubMed] [Google Scholar]
- Spiess M., Handschin C. Deletion analysis of the internal signal-anchor domain of the human asialoglycoprotein receptor H1. EMBO J. 1987 Sep;6(9):2683–2691. doi: 10.1002/j.1460-2075.1987.tb02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. An internal signal sequence: the asialoglycoprotein receptor membrane anchor. Cell. 1986 Jan 17;44(1):177–185. doi: 10.1016/0092-8674(86)90496-4. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Szczesna-Skorupa E., Browne N., Mead D., Kemper B. Positive charges at the NH2 terminus convert the membrane-anchor signal peptide of cytochrome P-450 to a secretory signal peptide. Proc Natl Acad Sci U S A. 1988 Feb;85(3):738–742. doi: 10.1073/pnas.85.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Wilson C., Connolly T., Morrison T., Gilmore R. Integration of membrane proteins into the endoplasmic reticulum requires GTP. J Cell Biol. 1988 Jul;107(1):69–77. doi: 10.1083/jcb.107.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C., Gilmore R., Morrison T. Translation and membrane insertion of the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus. Mol Cell Biol. 1987 Apr;7(4):1386–1392. doi: 10.1128/mcb.7.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S. L., Walter P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988 Nov;7(11):3559–3569. doi: 10.1002/j.1460-2075.1988.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]