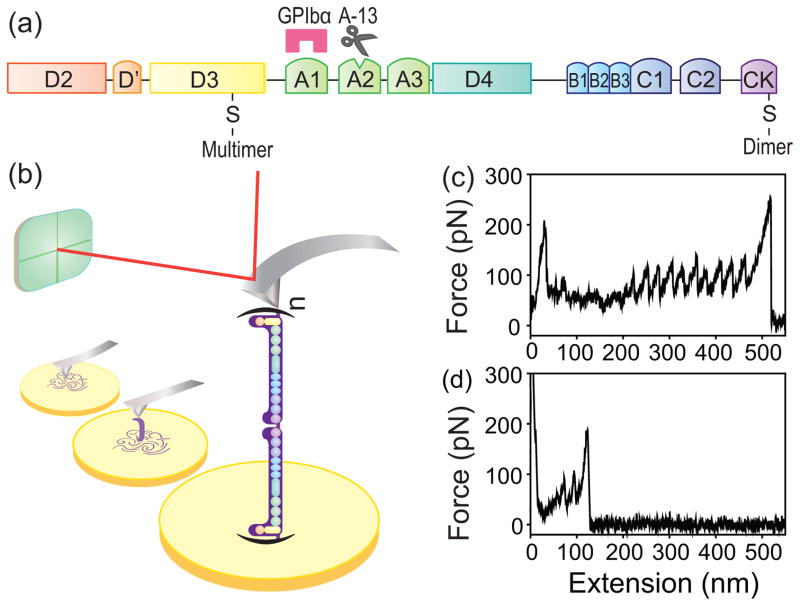
FIG. 1.
Multimeric VWF unfolding with AFM. (a) The domain organization of a VWF monomeric subunit, where A1 is the platelet GP Ib-IX-V receptor binding domain, A2 contains the cleavage site for ADAMTS-13, and D3 contains the binding site for the subendothelial matrix collagen. The locations of the disulfide bonds where the VWF connects to form dimers and multimers are presented. (b) Experimental setup of single-molecule pulling of a VWF multimer using AFM. A purified pVWF multimer, which is composed of n polymerized dimers of VWF, was pulled while the force was recorded. Typical force-extension curve of (c) a pVWF multimer, and (d) a pVWF dimer, pulled at a constant velocity of 1000 nm/s.